��Ŀ����
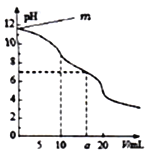
����Ŀ����1��25��ʱ��Ũ��Ϊ0.1 mo1��L-1��6����Һ����HCl����CH3COOH ��Ba(OH)2 ��Na2CO3 ��KCl ��NH4Cl��ҺpH��С�����˳��Ϊ_______________����д��ţ�
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh=_______ mo1��L-1 ��������С�����һλ����
��3��25��ʱ��pH=3�Ĵ����pH=11������������Һ�������Ϻ���Һ��________(������������������������������)����д����Һ������Ũ�ȼ��һ����ʽ��________________________________��
��4��25��ʱ����mmol/L�Ĵ����nmol/L������������Һ�������Ϻ���Һ��pH=7������Һ��c(CH3COO-)+c(CH3COOH)= _____________��m��n�Ĵ�С��ϵ��m_____n(����>����=������<��)��
��5����300mL1 mo1��L-1��NaOH��Һ���ձ�״����4.48LCO2ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ_________________________________________��
���𰸡� ��<��<��<��<��<�� 5.9��10-10 ���� (H+)+(Na+)=(CH3COO-)+C(OH-) ![]() mol/L > c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+)
mol/L > c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+)
��������������Ҫ��������ˮ�⡣
��1��25��ʱ��Ũ��Ϊ0.1 mo1��L-1��6����Һ����HCl��ȫ�������H+����CH3COOHС���ֵ������H+����Ba(OH)2��ȫ�������OH-����Na2CO3ˮ�����OH-����ˮ��̶�С����KCl��Һ�����ԣ�pH=7����NH4Clˮ�����H+����ˮ��̶�С�ڢ�CH3COOH�ĵ���̶ȣ�������ҺpH��С�����˳��Ϊ��<��<��<��<��<����
��2��25��ʱ������ĵ��볣��Ka=1.7��10-5mol/L������¶���CH3COONa��ˮ��ƽ�ⳣ��Kh=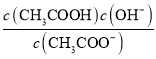 =
=![]() 5.9��10-10mo1��L-1��
5.9��10-10mo1��L-1��
��3��25��ʱ��pH=3�Ĵ����pH=11������������Һ�������Ϻ�ʣ��������ᣬ��Һ�����ԣ���Һ�е���غ���(H+)+(Na+)=(CH3COO-)+C(OH-)��
��4��c(CH3COO-)+c(CH3COOH)= ![]() mol/L������������������ǡ����ȫ��Ӧ�γɴ�������Һ���������ˮ��ʹ��Һ�ʼ��ԣ�����Һ��pH=7ʱ��ʣ����ᣬm>n��
mol/L������������������ǡ����ȫ��Ӧ�γɴ�������Һ���������ˮ��ʹ��Һ�ʼ��ԣ�����Һ��pH=7ʱ��ʣ����ᣬm>n��
��5����300mL1mo1��L-1��NaOH��Һ(����0.3molNaOH)���ձ�״����4.48L��0.2molCO2ʱ��3OH��+2CO2![]()
![]() +
+![]() +H2O���γɵ�Ũ�ȵ�NaHCO3��Na2CO3�Ļ����Һ��
+H2O���γɵ�Ũ�ȵ�NaHCO3��Na2CO3�Ļ����Һ�� ![]() ��
��![]() ˮ��ʹ��Һ�ʼ��ԣ����ǵ�ˮ��̶Ⱥ�С����ˮ��̶ȣ�
ˮ��ʹ��Һ�ʼ��ԣ����ǵ�ˮ��̶Ⱥ�С����ˮ��̶ȣ� ![]() <
<![]() ����ˣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊc(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+)��
����ˣ�������Һ�и�����Ũ���ɴ�С��˳��Ϊc(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+)��