ЬтФПФкШн
ЁОЬтФПЁПжаЙњЪзИіПеМфЪЕбщЪвЁЊЁЊЁАЬьЙЌвЛКХЁБЕФЙЉЕчЯЕЭГжаЃЌгадйЩњЧтбѕШМСЯЕчГиЃЈRFCЃЉЃЌЙЄзїдРэШчЯТЭМЫљЪОЃЌaЁЂbЁЂcЁЂdОљЮЊPtЕчМЋЁЃ
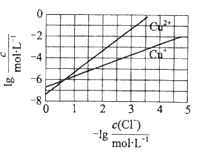
ЯТСаЫЕЗЈе§ШЗЕФЪЧ
A. BЧјЕФOHЭЈЙ§ИєФЄЯђaЕчМЋвЦЖЏЃЌAЧјpHдіДѓ
B. cЪЧе§МЋЃЌЕчМЋЩЯЕФЕчМЋЗДгІЮЊЃК2H++2e![]() H2Ёќ
H2Ёќ
C. ЭМжагвЙмжаЕФOHЭЈЙ§ИєФЄЯђcЕчМЋвЦЖЏЃЌdЕчМЋЩЯЗЂЩњбѕЛЏЗДгІ
D. ЕБга1 molЕчзгзЊвЦЪБЃЌБъзМзДПіЯТЃЌbЕчМЋРэТлЩЯВњЩњЦјЬхYЕФЬхЛ§ЮЊ11.2 L
ЁОД№АИЁПD
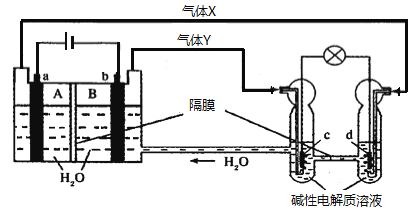
ЁОНтЮіЁПвРОнЭМЪОжЊзѓБпзАжУЪЧЕчНтГиЃЌгвБпзАжУЪЧдЕчГиЃЌabЕчМЋЪЧЕчНтГиЕФЕчМЋЃЌгЩЕчдДХаЖЯaЮЊбєМЋВњЩњЕФЦјЬхЪЧбѕЦјЃЌbЮЊвѕМЋВњЩњЕФЦјЬхЪЧЧтЦјЃЛcdЕчМЋЪЧдЕчГиЕФе§ИКМЋЃЌcЪЧИКМЋЃЌdЪЧе§МЋЃЛЕчНтГижаЕФЕчМЋЗДгІЮЊЃКaЕчМЋЮЊбєМЋЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃК4OH--4e-=2H2O+O2ЁќЃЛbЕчМЋЮЊвѕМЋЕУЕНЕчзгЗЂЩњЛЙдЗДгІЃК4H++4e-=2H2ЁќЃЛдЕчГижаЪЧМюадШмвКЃЌЕчМЋЗДгІЮЊЃКcЮЊИКМЋЪЇЕчзгЗЂЩњбѕЛЏЗДгІЃК2H2-4e-+4OH-=2H2OЃЛdЕчМЋЮЊе§МЋЕУЕНЕчзгЗЂЩњЛЙдЗДгІЃКO2+2H2O+4e-=4OH-ЁЃAЃЎaЮЊбєМЋЃЌЕчНтЪБвѕРызгЯђбєМЋвЦЖЏЃЌЗЂЩњ4OH--4e-=2H2O+O2ЁќЃЌДйНјЫЎЕФЕчРыЩњГЩH+ЃЌpHМѕаЁЃЌЙЪAДэЮѓЃЛBЃЎИљОнЩЯЪіЗжЮіЃЌcЪЧИКМЋЃЌЙЪBДэЮѓЃЛCЃЎЭМжагвЙмжаЕФOH-ЭЈЙ§ИєФЄЯђИКМЋвЦЖЏЃЌМДЯђcЕчМЋвЦЖЏЃЌdЮЊе§МЋЃЌЗЂЩњЛЙдЗДгІЃЌЙЪCДэЮѓЃЛDЃЎbЮЊвѕМЋЃЌЕБга1molЕчзгзЊвЦЪБЩњГЩ0.5molЧтЦјЃЌБъзМзДПіЯТЕФЬхЛ§ЮЊ11.2 LЃЌЙЪDе§ШЗЃЛЙЪбЁDЁЃ

ЁОЬтФПЁПЯжгаГЃЮТЯТЕФЫФжжШмвКЃЈШчЯТБэЃЉЃК
Ђй | Ђк | Ђл | Ђм | |
ШмвК | АБЫЎ | ЧтбѕЛЏФЦШмвК | ДзЫс | бЮЫс |
pH =11 | pH =11 | C=0.002mol/L | C=0.002mol/L |
ЯТСагаЙиа№Ъіжае§ШЗЕФЪЧ
A. ЗжБ№МгЫЎЯЁЪЭ10БЖЃЌЫФжжШмвКЕФpHЃКЂй>Ђк>Ђм>Ђл
B. дкЂмЁЂЂкСНжжШмвКЕШЬхЛ§ЛьКЯЃЌЫљЕУШмвКpH=3
C. ЫФжжШмвКгЩЫЎЕчРыГіЧтРызгЕФХЈЖШДгДѓЕНаЁЕФЫГађЪЧЂлЃОЂйЃОЂкЃОЂм
D. НЋЂйЁЂЂмСНжжШмвКЕШЬхЛ§ЛьКЯЃЌШмвКжаc(OHЃ) ЃОc(H+ )