��Ŀ����
5���±��г���A-R9��Ԫ�������ڱ��е�λ��| ����/�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
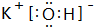 ������������ˮ����������ǿ������HClO4���⻯���ȶ���������CH4
������������ˮ����������ǿ������HClO4���⻯���ȶ���������CH4��2��DԪ�ص�����������Ӧ��ˮ������AԪ�ص�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2 H2O
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��Mg�����Ӱ뾶˳��Ϊ��K+��Na+��Mg2+
��4��F������⻯��ĵ���ʽ
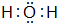 �����⻯���ڳ����¸�B������Ӧ�Ļ�ѧ����ʽ��2K+2 H2O=2KOH+H2�������ҳ̶ȴ��ڣ�����ڡ�С�ڡ����ڣ�A��
�����⻯���ڳ����¸�B������Ӧ�Ļ�ѧ����ʽ��2K+2 H2O=2KOH+H2�������ҳ̶ȴ��ڣ�����ڡ�С�ڡ����ڣ�A����5��CԪ�ظ�GԪ���γɵĻ�����ĵ���ʽ��
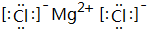 ���õ���ʽ��ʾ���γɹ���Ϊ
���õ���ʽ��ʾ���γɹ���Ϊ ��
����6��GԪ�غ�HԪ�أ�AԪ�غ�BԪ�غ˵����֮��ֱ�Ϊ18��8��
���� ��Ԫ�������ڱ���λ�ã���֪AΪNa��BΪKԪ�أ�CΪMg��DΪAl��EΪCԪ�أ�FΪOԪ�أ�GΪCl��HΪBr��RΪAr��
��1��ϡ�����廯ѧ��������ã�ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ��������ǿ��ΪKOH��������ǿ��Ϊ������ǽ�����Խ�����⻯���ȶ���Խ�
��2��DԪ�ء�AԪ�ص�����������Ӧ��ˮ����ֱ�ΪAl��OH��3��NaOH�����߷�Ӧ����ƫ��������ˮ��
��3��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
��4��F������⻯��ΪH2O��������K��ˮ��Ӧ������������������������������Խǿ��������ˮ��ӦԽ���ң�
��5��CԪ�ظ�GԪ���γɵĻ�����ΪMgCl2����þ�����������ӹ��ɣ�
��6��GԪ�غ�HԪ�غ˵����֮��Ϊ������������Ԫ��������AԪ�غ�BԪ�غ˵����֮��Ϊ������������Ԫ��������
��� �⣺��Ԫ�������ڱ���λ�ã���֪AΪNa��BΪKԪ�أ�CΪMg��DΪAl��EΪCԪ�أ�FΪOԪ�أ�GΪCl��HΪBr��RΪAr��
��1��ϡ������Ar�Ļ�ѧ��������ã�ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ��������Ԫ����K�Ľ�������ǿ��������ǿ��ΪKOH������ʽΪ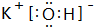 ��������ǿ��ΪHClO4���ǽ���Ԫ����C�ķǽ�������������CH4�ȶ�����
��������ǿ��ΪHClO4���ǽ���Ԫ����C�ķǽ�������������CH4�ȶ�����
�ʴ�Ϊ��Ar��K��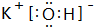 ��HClO4��CH4��
��HClO4��CH4��
��2��DԪ�ء�AԪ�ص�����������Ӧ��ˮ����ֱ�ΪAl��OH��3��NaOH�����߷�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2 H2O��
�ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2 H2O��
��3��ͬ�����������ԭ�Ӱ뾶��С��ͬ�������϶���ԭ�Ӱ뾶����ԭ�Ӱ뾶��K��Na��Mg�����Ӳ�ṹ��ͬ���˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ�����Ӱ뾶��K+��Na+��Mg2+��
�ʴ�Ϊ��K��Na��Mg��K+��Na+��Mg2+��
��4��F������⻯��ΪH2O������ʽΪ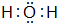 ��������K��ˮ��Ӧ��������������������������Ӧ����ʽΪ��2K+2 H2O=2KOH+H2����K�Ľ����Ա�Naǿ����K������ˮ��Ӧ�����ң�
��������K��ˮ��Ӧ��������������������������Ӧ����ʽΪ��2K+2 H2O=2KOH+H2����K�Ľ����Ա�Naǿ����K������ˮ��Ӧ�����ң�
�ʴ�Ϊ��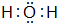 ��2K+2 H2O=2KOH+H2�������ڣ�
��2K+2 H2O=2KOH+H2�������ڣ�
��5��CԪ�ظ�GԪ���γɵĻ�����ΪMgCl2������ʽΪ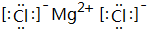 ��þ�����������ӹ��ɣ��õ���ʽ��ʾ���γ�Ϊ��
��þ�����������ӹ��ɣ��õ���ʽ��ʾ���γ�Ϊ�� ��
��
�ʴ�Ϊ��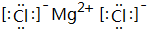 ��
�� ��
��
��6��GԪ�غ�HԪ�غ˵����֮��Ϊ������������Ԫ�����������˵�������18��AԪ�غ�BԪ�غ˵����֮��Ϊ������������Ԫ�������������ߺ˵�������8��
�ʴ�Ϊ��18��8��
���� ���⿼��Ԫ����������Ԫ�����ڱ����ۺ�Ӧ�ã���Ҫѧ�������������ڱ��Ľṹ�����ضԻ�ѧ����Ŀ��飬ע�����յ���ʽ��ʾ��ѧ�������ʵ��γɣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ����C���� | B�� | �������������Сһ�� | ||
| C�� | ��������������䣬����N2 | D�� | ��������������䣬����H2O��g�� |
| A�� | ��2 minĩ�ķ�Ӧ���ʣ���B��ʾ�� 0.3 mol/��L•min�� | |
| B�� | ��A��ʾ�ķ�Ӧ������0.4 mol/��L•min�� | |
| C�� | ����������Ҳͬʱ���д˷�Ӧ������ͬʱ�������D��ʾ��������0.2 mol/��L•min�������������з�Ӧ���ʸ��� | |
| D�� | �Լ����������¶ȣ�������Ӧ���ʽ���С |
| A�� | Ҫ����ij�������е���Ԫ�أ����Լ���NaOH��Һ���ȣ���ȴ�����AgNO3��Һ���۲�����dz��ɫ�������� | |
| B�� | ʵ������ȡ��ϩʱ�������д̼�����ζ�����������˵�������Ҵ�������Ϊ��ȩ | |
| C�� | �����ܷ���������Ӧ������һ����ȩ�������еļ��� | |
| D�� | ������Һ��ͨ������Ķ�����̼����ʱ�������ﲻ������̼���� |
| A�� | ��1 mol NaHSO4�����У�����������Ϊ2NA | |
| B�� | 1 mol C4H10�����й��ۼ�����Ϊ13NA | |
| C�� | 0.5 mol•L-1 Ba��NO3��2��Һ�У�NO${\;}_{3}^{-}$����ĿΪNA | |
| D�� | �κ������£�20 L N2���еķ�������������ΪNA |
| Ԫ�ر�� | Ԫ��������ԭ�ӣ�����ӣ��ṹ |
| T | �����������Ǵ�����������3�� |
| X | �����£�����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ�����⻯���ˮ��Һ�Լ��� |
| Y | M���K����1������ |
| Z | ��3����Ԫ�صļ������а뾶��С�ģ�������������� |
 ��
����2��Ԫ��Y��Ԫ��Z��ȣ������Խ�ǿ����Na����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����cd������ţ���
a��Y���ʵ��۵��Z���ʵ� b��Y�Ļ��ϼ۱�Z��
c��Y������ˮ��Ӧ��Z���ʾ��� d��Y����������ˮ����ļ��Ա�Zǿ
��3��T��X��Y��Z��������Ԫ�����γɼ������Ӽ����зǼ��Թ��ۼ��Ļ����д���û�����Ļ�ѧʽΪNa2O2��
��4��Ԫ��T����Ԫ����ԭ�Ӹ�����1��1�γɵĻ�����ĵ���ʽΪ
 ��Ԫ��Z����������ˮ������Ԫ��Y������������ˮ�������Һ���Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��Ԫ��Z����������ˮ������Ԫ��Y������������ˮ�������Һ���Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O�� 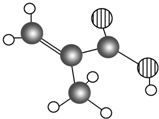 ij��������Ʒֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӣ�ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ���
ij��������Ʒֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӣ�ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ��� ��
��