��Ŀ����
15�����в��ֶ�����Ԫ�ص�������ԭ�ӣ�����ӣ��ṹ���±���| Ԫ�ر�� | Ԫ��������ԭ�ӣ�����ӣ��ṹ |
| T | �����������Ǵ�����������3�� |
| X | �����£�����Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ�����⻯���ˮ��Һ�Լ��� |
| Y | M���K����1������ |
| Z | ��3����Ԫ�صļ������а뾶��С�ģ�������������� |
 ��
����2��Ԫ��Y��Ԫ��Z��ȣ������Խ�ǿ����Na����Ԫ�ط��ű�ʾ�������б�������֤����һ��ʵ����cd������ţ���
a��Y���ʵ��۵��Z���ʵ� b��Y�Ļ��ϼ۱�Z��
c��Y������ˮ��Ӧ��Z���ʾ��� d��Y����������ˮ����ļ��Ա�Zǿ
��3��T��X��Y��Z��������Ԫ�����γɼ������Ӽ����зǼ��Թ��ۼ��Ļ����д���û�����Ļ�ѧʽΪNa2O2��
��4��Ԫ��T����Ԫ����ԭ�Ӹ�����1��1�γɵĻ�����ĵ���ʽΪ
 ��Ԫ��Z����������ˮ������Ԫ��Y������������ˮ�������Һ���Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
��Ԫ��Z����������ˮ������Ԫ��Y������������ˮ�������Һ���Ӧ�����ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O��
���� ������Ԫ���У�TԪ��ԭ�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����TΪCԪ�أ�
�����£�XԪ�ص���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ�����⻯���ˮ��Һ�Լ��ԣ���XΪNԪ�أ�
YԪ��ԭ��M���K����1�����ӣ�M�������Ϊ1����YΪNa��
��3����Ԫ��Z�ļ������а뾶��С�ģ�������������ԣ���ZΪAl��
�ݴ˽��
��� �⣺������Ԫ���У�TԪ��ԭ�������������Ǵ�����������3����ԭ��ֻ����2�����Ӳ㣬����������Ϊ6����TΪ��Ԫ�أ������£�XԪ�ص���Ϊ˫ԭ�ӷ��ӣ������к���3�Թ��õ��Ӷԣ�����⻯���ˮ��Һ�Լ��ԣ���XΪ��Ԫ�أ�YԪ��ԭ��M���K����1�����ӣ�M�������Ϊ1����YΪNa����3����Ԫ��Z�ļ������а뾶��С�ģ�������������ԣ���ZΪAl��
��1��TΪOԪ�أ���ԭ�Ӻ�����2�����Ӳ㣬K��L��������ֱ�Ϊ2��6��ԭ�ӽṹʾ��ͼΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��ͬ��������������Լ������ʽ�����Na��Al���ȽϽ���ǿ���ķ����У���ˮ���ᷴӦ�ľ��ҳ̶ȡ�������������ˮ����ļ���ǿ�����������˳����ȣ��뵥���۷е㡢Ԫ�ػ��ϼ��أ�����ѡc��d��
�ʴ�Ϊ��Na��c��d��
��3��O��N��Na��Al��������Ԫ�����γɼ������Ӽ����зǼ��Թ��ۼ��Ļ�����û�����Ļ�ѧʽΪNa2O2��
�ʴ�Ϊ��Na2O2��
��4��Ԫ��T����Ԫ����ԭ�Ӹ�����1��1�γɵĻ�����ΪH2O2������ʽΪ ��Ԫ��Z��Y����������ˮ����ֱ�ΪAl��OH��3��NaOH�����߷�Ӧ������ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
��Ԫ��Z��Y����������ˮ����ֱ�ΪAl��OH��3��NaOH�����߷�Ӧ������ƫ��������ˮ����Ӧ���ӷ���ʽΪ��Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ�� ��Al��OH��3+OH-=AlO2-+2H2O��
��Al��OH��3+OH-=AlO2-+2H2O��
���� ���⿼��λ�ýṹ���ʹ�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ���ע���������ս����ԡ��ǽ�����ǿ���Ƚ�ʵ����ʵ��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�| ����/�� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
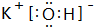 ������������ˮ����������ǿ������HClO4���⻯���ȶ���������CH4
������������ˮ����������ǿ������HClO4���⻯���ȶ���������CH4��2��DԪ�ص�����������Ӧ��ˮ������AԪ�ص�����������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2 H2O
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������ΪK��Na��Mg�����Ӱ뾶˳��Ϊ��K+��Na+��Mg2+
��4��F������⻯��ĵ���ʽ
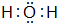 �����⻯���ڳ����¸�B������Ӧ�Ļ�ѧ����ʽ��2K+2 H2O=2KOH+H2�������ҳ̶ȴ��ڣ�����ڡ�С�ڡ����ڣ�A��
�����⻯���ڳ����¸�B������Ӧ�Ļ�ѧ����ʽ��2K+2 H2O=2KOH+H2�������ҳ̶ȴ��ڣ�����ڡ�С�ڡ����ڣ�A����5��CԪ�ظ�GԪ���γɵĻ�����ĵ���ʽ��
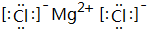 ���õ���ʽ��ʾ���γɹ���Ϊ
���õ���ʽ��ʾ���γɹ���Ϊ ��
����6��GԪ�غ�HԪ�أ�AԪ�غ�BԪ�غ˵����֮��ֱ�Ϊ18��8��
| ��� | ʵ������ | ʵ��Ŀ�� |
| A | �ⶨ��ͬŨ�ȵ�NaClO��Һ��CH3COONa��Һ��pH | �Ƚ�HClO��CH3COOH������ǿ�� |
| B | ��Mg��OH��2��Һ�еμ�����0.1mol/LFeCl3��Һ | �Ƚ�Mg��OH��2��Fe��OH��3���ܽ�� |
| C | ��������ȫ��ͬ�ҳ���NO2���ܱ���ƿ���ֱ��������ˮ����ˮ�� | ̽���¶ȶԻ�ѧƽ��״̬��Ӱ�� |
| D | ��ͬ���ͬŨ��H2O2��Һ�У��ֱ����1molͨŨ�ȵ�CuSO4��FeCl3��Һ | �Ƚ�Cu2+��Fe3+��H2O2�ֽ����ʵ�Ӱ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | �Ը÷�Ӧ��ϵ���� | B�� | ��������NaCl��Һ | ||
| C�� | ����пƬ������п�� | D�� | ��������1 mol•L-1���� |
| A�� | ��CH3COOH��Һ�м�ˮ��c��OH������c��H+�������� | |
| B�� | ���ʵ���Ũ�Ⱦ�Ϊ0.1mol•L��1��CH3COOH��Һ��NaOH��Һ�������ϣ�c��Na+����c��CH3COO���� | |
| C�� | ijŨ�ȵ��Ȼ����Һ�д���c��NH4+����c��Cl������c��H+����c��OH���� | |
| D�� | ��pH=4���Ȼ����Һ��c��H+��+c��NH4+��=c��Cl����+c��OH���� |
| A�� | Mg2+��Al3+��Cl-��Ne | B�� | Na+��F-��S2-��Ar | ||
| C�� | K+��Ca2+��S2-��Ar | D�� | Mg2+��Na+��Cl-��S2- |
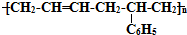 �ĸ߾���䵥���ǣ�������
�ĸ߾���䵥���ǣ��������ٱ���ϩ �ڶ�ϩ ��1��3-����ϩ �ܱ�Ȳ �ݱ���ϩ��
| A�� | �٢� | B�� | �ܢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | Na+��Ba2+��Cl-��SO42- | B�� | Ca2+��HCO3-��Cl-��K+ | ||
| C�� | Mg2+��Ag+��NO3-��Cl- | D�� | H+��Cl-��Na+��CO32- | ||
| E�� | SO32-��Ba2+��H+��NO3- |