��Ŀ����
18���±���Ԫ�����ڱ���ǰ�����ڣ��ش��������⣺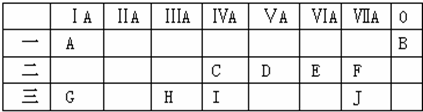
��1�������ڰ뵼����ϵ�Ԫ����Si ����Ԫ�ط��ţ�������Ԫ�����ڱ��е�λ��Ϊ��3���ڢ�A�壻
��2��A��G����Ԫ�طֱ���EԪ�ض����γ����ֻ���������������ӻ��������Na2O��Na2O2��д��ѧʽ����ͬ�������ڹ��ۻ��������H2O��H2O2�������ֻ������к��зǼ��Լ��Ļ�������H2O2��Na2O2��
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ����NaOH���������������������Al��OH��3���û�ѧʽ��ʾ����
��4��ֻ����A��C����Ԫ�صĻ��������������Щ����������Է���������С���Ǽ��飬�û�������ӵĿռ乹�����������壻
��5������Ԫ��H��ԭ�ӽṹʾ��ͼ
 ��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����
��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3������6����Ԫ��A��D��J��ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ�����Ļ�ѧʽΪNH4Cl��
���� ��A��I��Ԫ�����ڱ��е�λ�ÿ�֪��A��H��B��He��C��C��D��N��E��O��F��F��G��Na��H��Al��I��Si��JΪCl��
��1������Ԫ���У����ڰ뵼����ϵ�Ϊ�裬��ԭ������Ϊ14��������Ӳ���Ϊ3������㺬��4�����ӣ��ݴ��ж���λ�ã�
��2��H��NaԪ�ص��ܹ�����Ԫ��д������������ֱ�Ϊˮ��˫��ˮ�������ơ��������ƣ�Ȼ��������ӻ�������ۻ�����ĸ�����еĻ�ѧ�����ͽ����жϣ�
��3���ǽ�����Խǿ��������������Ӧˮ����ļ���Խǿ����������Ϊ�����������
��4��ֻ����C��HԪ�صĻ������Ϊ������Է���������С����Ϊ���飬����Ϊ��������ṹ��
��5��HΪ������ԭ�ӵĺ˵����=�����������=13���Ȼ���������������������Һ��Ӧ������������������
��6��A��D��J�ֱ�ΪH��N��Cl�����Ƿǽ���Ԫ�أ�������ɵ����ӻ�������һ������笠����ӣ���û�����Ϊ�Ȼ�泥�
��� �⣺��A��I��Ԫ�����ڱ��е�λ�ÿ�֪��A��H��B��He��C��C��D��N��E��O��F��F��G��Na��H��Al��I��Si��JΪCl��
��1������10��Ԫ���У������ڰ뵼����ϵ�ΪSi��������������Ϊ14��λ�����ڱ��е�3���ڢ�A�壬
�ʴ�Ϊ��Si����3���ڢ�A�壻
��2��AΪH��GΪNa��������OԪ�ض����γ����ֻ����Na2O��Na2O2��H2O��H2O2�����к������Ӽ��������ӻ��������Na2O��Na2O2�����ڹ��ۻ��������H2O��H2O2�������ֻ������к��зǼ��Լ��Ļ�������H2O2��Na2O2��
�ʴ�Ϊ��Na2O��Na2O2�� H2O��H2O2��H2O2��Na2O2��
��3������Ԫ���У���������ȷ��ΪNa��������������Ӧˮ����ļ�����ǿ��ΪNaOH��Al��OH��3Ϊ��������������ܹ����ᷴӦ������ǿ����Һ��Ӧ��
�ʴ�Ϊ��NaOH�� Al��OH��3��
��4��ֻ����A��C����Ԫ�صĻ�����������������������Է���������С���Ǽ��飬������ӵĿռ乹�����������壬
�ʴ�Ϊ���������飻�������壻
��5��Alԭ�ӵĺ˵���������������������13����ԭ�ӽṹʾ��ͼΪ�� ��
��
Ԫ��H��Ԫ��J��ɵĻ�����Ϊ�Ȼ������Ȼ�����ˮ��Һ�м��������ռ���Һ��Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3++3OH-=Al��OH��3����
�ʴ�Ϊ�� ��Al3++3OH-=Al��OH��3����
��Al3++3OH-=Al��OH��3����
��6��A��D��J�ֱ�ΪH��N��Cl�����߶��Ƿǽ���Ԫ�أ�������ɵ����ӻ�������һ������笠����ӣ���û�����Ϊ��NH4Cl��
�ʴ�Ϊ��NH4Cl��
���� ���⿼����Ԫ�����ڱ���Ԫ�������ɵ��ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷԪ�����ڱ��ṹ��Ԫ������������Ϊ���ؼ���������ؿ���ѧ���ķ������������Ӧ�û���֪ʶ��������

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�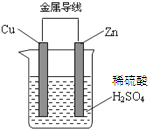
| A�� | пƬ������ | |
| B�� | ͭƬ�Ϸ����ķ�ӦΪ Cu-2e-�TCu2+ | |
| C�� | ������ͭƬͨ����������пƬ | |
| D�� | ��װ���ܹ�����ѧ��ת��Ϊ���� |
| A�� | Һ�ȡ�����衢���������ڵ��� | B�� | ���ᡢ���ᡢ���Ӿ�����ǿ����� | ||
| C�� | CO2��CCl4�������ʾ������л��� | D�� | CuO��MgO��Na2O2�����ڼ��������� |
| A�� | ��֬��ˮ�����Ϊ������ | B�� | ����ˮ������ղ����������� | ||
| C�� | �����£������������ɫ | D�� | �������ս�ʱû��������ζ |
 ����ȼ�ϵ����һ�ֽ���ѧ��ת��Ϊ���ܵĸ�Ч�������Ѻõķ���װ�ã��õ�صĹ�����ͼ��ʾ�����з����жϴ�����ǣ�������
����ȼ�ϵ����һ�ֽ���ѧ��ת��Ϊ���ܵĸ�Ч�������Ѻõķ���װ�ã��õ�صĹ�����ͼ��ʾ�����з����жϴ�����ǣ�������| A�� | a��Ϊ������b��Ϊ���� | |
| B�� | ��������������Ӧ | |
| C�� | �����ĵ缫��ӦΪO2+2H2O+2e-�T4OH- | |
| D�� | �����ĵ缫��ӦΪH2+2OH--2e-�T2H2O |
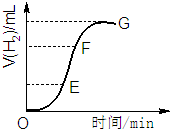 �ò�����п����2mol/L���ᷴӦ��ȡ�������壬��ش�
�ò�����п����2mol/L���ᷴӦ��ȡ�������壬��ش� ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��