��Ŀ����
3����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ��������ЧӦ����Ҫ���壮B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ����1��A��B��C�ĵ�һ��������С�����˳��ΪC��O��N��
��2��B���⻯����ӵ����幹���������Σ�������ԭ�Ӳ�ȡsp3�ӻ���
��3��д��������AC2�ĵ���ʽ
 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪN2O����4��E�ĺ�������Ų�ʽ��1s22s22p63s23p63d54s1��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
���� ������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�CΪOԪ�أ�AC2Ϊ��������ЧӦ����Ҫ���壬ӦΪCO2����AΪCԪ�أ���BΪNԪ�أ�E��ԭ������Ϊ24��ӦΪCrԪ�أ���϶�ӦԪ�صĵ��ʺͻ�����������Լ���ĿҪ��ɽ����⣮
��� �⣺������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�CΪOԪ�أ�AC2Ϊ��������ЧӦ����Ҫ���壬ӦΪCO2����AΪCԪ�أ���BΪNԪ�أ�E��ԭ������Ϊ24��ӦΪCrԪ�أ�
��1��ͬ����Ԫ�ص�һ�����ܴ���������������ƣ��ʵ����ܴ�СΪC��O��N���ʴ�Ϊ��C��O��N��
��2��BΪNԪ�أ���Ӧ���⻯��ΪNH3������������ԭ���γ�3���ļ�����1���µ��Ӷԣ�Ϊsp3�ӻ����ռ乹��Ϊ�����Σ��ʴ�Ϊ�������Σ�sp3��
��3��AC2ΪCO2��Ϊ���ۻ��������ʽΪ ��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ��
��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ�� ��N2O��
��N2O��
��4��EΪCrԪ�أ�E�ĺ�������Ų�ʽ��1s22s22p63s23p63d54s1����ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬��֪�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d54s1��[Cr��NH3��4��H2O��2]Cl3��
��5��N����ͼ�Ϊ-3��DΪMgԪ�أ���Ӧ����NH4NO3����Ӧ�ķ���ʽΪ4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
���� ���⿼��Ԫ���ƶ��⣬������ԭ�ӽṹ��Ԫ�������ɵĿ��飬Ϊ�߿��������ͣ��ƶϳ�Ԫ�ص������ǽ����Ĺؼ����ƶ�ʱע���ԭ�ӵĺ�������Ų��ص��Լ�Ԫ�ص���������Ϊͻ�ƿڽ�𣬱������һ���Ѷȣ�

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
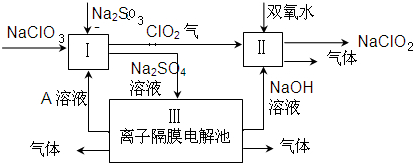
���������ν�ϵ�д�| A�� | Ư��¶���ڿ�����ʧЧ��ClO-+CO2+H2O�THClO+HCO3- | |
| B�� | ����з�̪�Ĺ�������Һ�бӱ��صμ���������ɫ��dz���ӽ���ʧ��2H++SiO32-�TH2SiO3�����壩 | |
| C�� | ��Na2S2O3��Һ��ͨ������������S2O32-+2Cl2+3H2O�T2SO32-+4Cl-+6H+ | |
| D�� | ��ǿ����Һ�д���������Fe��OH��3��Ӧ����Na2FeO4��3ClO-+Fe��OH��3�TFeO42-+3Cl-+H2O+4H+ |
| A�� | A��B��C�ķ�����֮��Ϊ1��3��2 | |
| B�� | A��B��C��Ũ����� | |
| C�� | ��λʱ������ nmol A��ͬʱ���� 3nmol B | |
| D�� | ����C��������C�ֽ��������� |
| A�� | CH4���ӵĽṹʽ��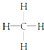 | B�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | ||
| C�� | F-�ṹʾ��ͼ��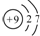 | D�� | �����ӵĵ���ʽ��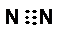 |
| A�� | ���ȩ������ԭ����Ư�ۡ���������������ͬ | |
| B�� | 1 mol ���ȩ���ӿɱ�1 mol Cu��OH��2��ȫ���� | |
| C�� | CH3CH�TCHCH2COOH�����ȩ��Ϊͬ���칹�� | |
| D�� | 10g���ȩ��ȫȼ��������0.6 mol O2 |
| A�� | Fe2O3 | B�� | MnO2 | C�� | CaO | D�� | V2O5 |
| A�� | ú�ĸ����ʯ�͵ķ��������ѧ�仯 | |
| B�� | �����ʺ����Ƕ����ڸ߷��ӻ����һ�������¶���ˮ�� | |
| C�� | ��ϩˮ�����Ҵ���ԭ��������Ϊ�ٷ�֮�٣�������ɫ��ѧԭ�� | |
| D�� | ��������ά�ػ�Ϊͬ���칹�� |
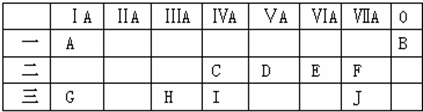
 ��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����
��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����