��Ŀ����
1��ijУ��ѧ��ȤС��Ϊ̽������Ũ����ķ�Ӧ�������ͼ1��ͼ2��ʾװ�ý���ʵ�飮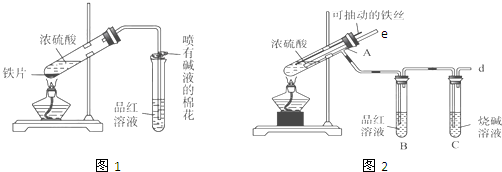
��1����˵����SO2���������ʵ��������B��Ʒ����Һ��ɫ��
��2��ͼ2�е�����e����Ҫ����Ϊֹͣ����ʱ���ܷ�ֹ������ƽ��ѹǿ��
��3������װ����ͼ2�е�NaOH��Һ������SO2β������ֹ��Ⱦ���罫�����Ϊ����KMnO4��Һ��ͬ�����ԴﵽĿ�ģ���д������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4��
��4���Ա�����ʵ��װ�ã����ѷ���ͼ2װ�ó����ܸ��õ������ж�����SO2��ֹ����Ⱦ�����⣬����һ���dz����Ե��ŵ㣬����Ϊ�DZ��ڿ��Ʒ�Ӧ�ķ�����ֹͣ��
��5����Ӧһ��ʱ���ֹͣ��Ӧ������ȴ���ý�ͷ�ι���ȡA�Թ��е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ���ֻ����Fe3+����ֻ����Fe2+������Fe3+����Fe2+��
Ϊȷ����Һ�ijɷ֣�ѡ�������Լ���
A��ϡHCl��Һ B��ϡ���� C��KSCN��Һ D������KMnO4��ҺE��NaOH��Һ F��H2O2��Һ
�����������ص�ʵ��̽����
| ʵ�鲽�� | ʵ�������� |
| 1��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ | ��1�����û�й۲쵽��Һ��ɫ�����Ա仯����˵��������� ��2������۲쵽��Һ��ɫת��Ϊ��ɫ����˵����Һ�д���Fe3+������������ |
| 2�� ��ȡһ֧�ྻ���Թܣ���1-2mL������Һ���ý�ͷ�ι���εμ����Ը��������Һ | ����μ����Ը��������Һ����Һ���Ϻ�ɫ��ɫ����˵����Һ�к����������ӣ�˵�������������μ����Ը��������Һ����Һ�Ϻ�ɫ���ʣ���˵����Һ��û���������ӣ�˵��������� |
���� ��1�����������ܹ�ʹƷ����Һ��ɫ���ݴ˿��ж��Ƿ����ɶ�������
��2��������e�������ͨ����ƽ��ѹǿ��ֹ���������ã�
��3�����Ը��������Һ����ǿ�����ԣ��ܹ������������������ԭ��Ӧ�������ᡢ�����̺�ˮ��
��4��ͼ2װ����Fe˿���Գ鶯���ܿ��Ʒ�Ӧ�ķ�����ֹͣ��
��5�������������軯�ط�Ӧ��������Ѫ��ɫ����Һ���ô����������������ӣ����������ӵļ����ǵ����仹ԭ�ԣ��������ӱ��������������Ȼ�����������軯�ؽ���ȷ����
��� �⣺��1�������������Ư���ԣ�����Ʒ����Һ��ɫ������B��Ʒ����Һ��ɫ��֤���ж����������ɣ�
�ʴ�Ϊ��B��Ʒ����Һ��ɫ��
��2��������e�������ͨ����ƽ��ѹǿ����ֹ���������ã�
�ʴ�Ϊ��ֹͣ����ʱ���ܷ�ֹ������ƽ��ѹǿ���������𰸾��ɣ���
��3������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽΪ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4��
�ʴ�Ϊ��2KMnO4+5SO2+2H2O=K2SO4+2MnSO4+2H2SO4��
��4����Ҫ�÷�Ӧ��ʼ������˿��һ������Ӵ�Ũ���ᣬҪ�뷴Ӧֹͣʱ������˿����Ũ���ᣬ�������ڿ��Ʒ�Ӧ�ķ�����ֹͣ��
�ʴ�Ϊ�����ڿ��Ʒ�Ӧ�ķ�����ֹͣ��
��5��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ�����û�й۲쵽��Һ��ɫ�����Ա仯��˵����Һ�в����������ӣ����������������۲쵽��Һ��ɫת��Ϊ��ɫ��˵����Һ�д��������ӣ��������������
��ȡһ֧�ྻ���Թܣ���1-2mL������Һ���ý�ͷ�ι���εμ����Ը��������Һ�����������ܹ��������������ӣ��������Ը��������Һ��ɫ������μ����Ը��������Һ����Һ���Ϻ�ɫ��ɫ����˵����Һ�к����������ӣ�˵�������������μ����Ը��������Һ����Һ�Ϻ�ɫ���ʣ���֤����Һ��û���������ӣ��Ӷ�˵���������
�ʴ�Ϊ��
| ʵ�鲽�� | ʵ�������� |
| ��1�����û�й۲쵽��Һ��ɫ�����Ա仯�� ��2������۲쵽��Һ��ɫת��Ϊ��ɫ�� | |
| 2����ȡһ֧�ྻ���Թܣ���1-2mL������Һ���ý�ͷ�ι���εμ����Ը��������Һ | ����μ����Ը��������Һ����Һ���Ϻ�ɫ��ɫ����˵����Һ�к����������ӣ�˵�������������μ����Ը��������Һ����Һ�Ϻ�ɫ���ʣ���˵����Һ��û���������ӣ�˵������� |
���� ����ͨ��Ũ��������ʣ����ؿ�������ʵ�鷽������������ۣ���Ŀ�ѶȽϴ���ȷ�����ӡ��������ӵļ��鷽��Ϊ���ؼ���ע����������ʵ�鷽�������ԭ�������ֿ�����ѧ���ķ�����������������ѧʵ��������

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�| A�� | 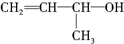 | B�� | CH2�TCH-CH3 | C�� | 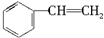 | D�� | 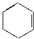 |
| A�� | �����ԣ�Na��Mg��Al | B�� | ���ȶ��ԣ�HCl��H2S��PH3 | ||
| C�� | ����ǿ����H2CO3��HSO4��HClO4 | D�� | �۵㣺Na��K��Rb |
| A�� | ����ŨHNO3��ŨH2SO4�����Ծ���ǿ�������¶������������������� | |
| B�� | ¶���ڿ����У���������Һ������������ | |
| C�� | �����¶�����ͭ�Ͽ췴Ӧ | |
| D�� | ¶���ڿ����У���������Һ��Ũ�ȶ���С |
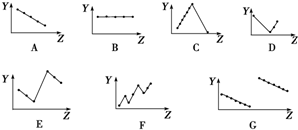
��֪����ZrO2�����ռӦ���ɿ�����ˮ��Na2ZrO3��Na2ZrO3���ᷴӦ����ZrO2+��
�ڲ��ֽ���������ʵ�������¿�ʼ��������ȫ������pH�����
| �������� | Fe3+ | Al3+ | ZrO2+ |
| ��ʼ����ʱpH | 1.9 | 3.3 | 6.2 |
| ������ȫʱpH | 3.2 | 5.2 | 8.0 |
��2��Ϊʹ��ҺI���������ӳ�����ȫ�����ð�ˮ��pH=a����a�ķ�Χ��5.2��6.2�������Ӱ�ˮ��pH=bʱ����������Ӧ�����ӷ���ʽΪZrO2++2NH3•H2O+H2O=Zr��OH��4��+2NH4+��
��3������ˢ�������Һ�м���CaCO3��ĩ�����ȣ��õ��������壮�÷�Ӧ�����ӷ���ʽΪ2NH4++CaCO3=Ca2++2NH3��+CO2��+H2O��
��4��Ϊ�õ�������ZrO2��Zr��OH��4��Ҫϴ�ӣ�����Zr��OH��4�Ƿ�ϴ�Ӹɾ��ķ�����ȡ���һ��ϴ��Һ�������еμ�ϡ���ᣬ�ٵμ���������Һ�����������ɣ���Zr��OH��4ϴ�Ӹɾ���
| A�� | ��ˮ������� | B�� | ��ˮ��Ͷ��һС������� | ||
| C�� | ��ˮ��ͨ������������� | D�� | ��ˮ�м�ʳ�ξ��� |
 ���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ������������[A12��SO4��3•18H2O]��
���÷���������Ҫ�ɷ�ΪAl��������Fe��Si�ȣ��ȿ���ȡ�л��ϳɴ���AlBr3�ֿ���ȡ��ˮ������������[A12��SO4��3•18H2O]��