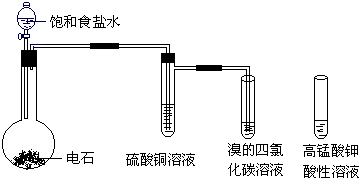
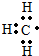��Ŀ����
12��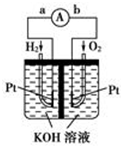 ����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش�
����ȼ�ϵ���Ƿ�����ɫ��ѧ��������ͷ���װ�ã���ͼΪ���ʾ��ͼ���õ�ص缫�����һ��ϸС�IJ��ۣ����������������ǿ�������ȶ�����ش���1������ȼ�ϵ�ص�����ת������Ҫ��ʽ�ǻ�ѧ��ת��Ϊ���ܣ��ڵ����е�����������Ϊ��a��b����a��b��ʾ����
��2��������ӦΪ2H2+4OH--4e-�T4H2O��������ӦΪ2H2O+O2+4e-�T4OH-��
��3���缫����Ʋ��۵���������缫��λ�������H2��O2�ķ��������ӿ�缫��Ӧ���ʣ�
���� ��1��ԭ����ǽ���ѧ��ת��Ϊ���ܵ�װ�ã�ԭ��طŵ�ʱ�����ӴӸ����ص�������������
��2��������ȼ��ʧ���ӷ�����ԭ��Ӧ�������������õ����������������ӣ�
��3���μӷ�Ӧ������Ũ������Ӧ���ʼӿ죮
��� �⣺��1����װ���ǰѻ�ѧ�����е�����ת��Ϊ���ܣ������ǻ�ѧ��ת��Ϊ���ܣ���ԭ����У�������ʧ���ӣ������ϵõ��ӣ����ӵ������ǴӸ������������������� ��a��b��
�ʴ�Ϊ����ѧ��ת��Ϊ���ܣ�a��b��
��2�����Ի����У��÷�Ӧ�и���������ʧ�������������ӣ��缫��ӦʽΪH2+2OH--2e-�T2H2O�������������õ����������������ӣ��缫��ӦʽΪ2H2O+O2+4e-�T4OH-���ʴ�Ϊ��2H2+4OH--4e-�T4H2O��2H2O+O2+4e-�T4OH-��
��3���缫����Ʋ��ۣ������˵缫��λ�������H2��O2�ķ�������ʹ��Ӧ���Ũ������Ӧ���ʼӿ죬
�ʴ�Ϊ������缫��λ�������H2��O2�ķ��������ӿ�缫��Ӧ���ʣ�
���� ���⿼�黯ѧ��Դ�Ĺ���ԭ������Ŀ�ѶȽϴ�ע��ԭ��ص缫��Ӧʽ����д��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
9������Ԫ��R����������Ӧˮ����Ļ�ѧʽΪH2RO3�������⻯��Ļ�ѧʽ�ǣ�������
| A�� | HR | B�� | H2R | C�� | RH3 | D�� | RH4 |
10����������;�㷺��������ָʾ���ʹ����Ʊ���һ������ˮ�ܿ�[��Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��]��ȡCoC2O4•2H2O����������ͼ1��
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
��1�����������м���Na2SO3��Ŀ���ǽ�Fe3+��Co3+��ԭ�������ӷ��ţ���
��2��NaClO3�������ǽ�����Һ�е�Fe2+������Fe3+����������Ԫ�ش�����ͻ��ϼۣ��÷�Ӧ�����ӷ���ʽΪClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
��3������ƽ���ƶ�ԭ��������Na2CO3��ʹ����Һ��Fe3+��Al3+ת�����������������ԭ��R3++3H2O?R��OH��3+3H+������̼���ƺ�H+��CO32-��Ӧ��ʹˮ��ƽ�����ƣ��Ӷ�����������
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��ʾ��
��Һ���м�����ȡ���������dz�ȥMn2+��ʹ����ȡ�����˵�pH��B��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5 D��5.0��5.5
��5�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪Ksp��MgF2��=7.35��10-11��Ksp��CaF2��=1.05��10-10�����������NaF��������Һ$\frac{c��M{g}^{2+}��}{c��C{a}^{2+}��}$=0.7��
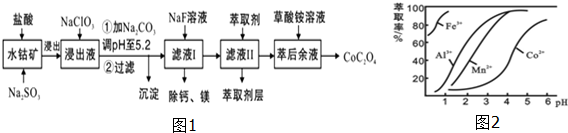
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ȫ������pH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��2��NaClO3�������ǽ�����Һ�е�Fe2+������Fe3+����������Ԫ�ش�����ͻ��ϼۣ��÷�Ӧ�����ӷ���ʽΪClO3-+6Fe2++6H+=6Fe3++Cl-+3H2O��
��3������ƽ���ƶ�ԭ��������Na2CO3��ʹ����Һ��Fe3+��Al3+ת�����������������ԭ��R3++3H2O?R��OH��3+3H+������̼���ƺ�H+��CO32-��Ӧ��ʹˮ��ƽ�����ƣ��Ӷ�����������
��4����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��ʾ��
��Һ���м�����ȡ���������dz�ȥMn2+��ʹ����ȡ�����˵�pH��B��
A��2.0��2.5 B��3.0��3.5 C��4.0��4.5 D��5.0��5.5
��5�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪Ksp��MgF2��=7.35��10-11��Ksp��CaF2��=1.05��10-10�����������NaF��������Һ$\frac{c��M{g}^{2+}��}{c��C{a}^{2+}��}$=0.7��

7������һ�ε��Ʒ�ֺܶ࣬����п�̵�����ճ������������ܴ��շϾ�п�̵�ز��������´��������Ի��MnO2����������Ʒ���乤��������ͼ��
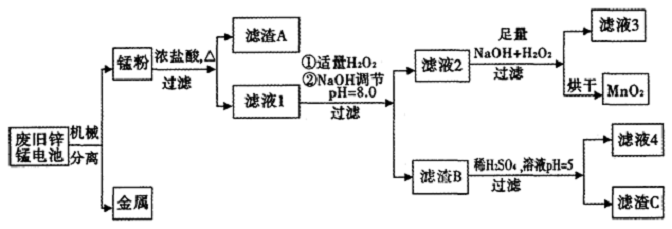
��֪�����̷ۡ�����Ҫ�ɷ���MnO2��Zn��OH��2��MnOOH��̼�ۣ��������������κ������Σ������£������������������pH���±���
��1������NaOH��Һ����pH=8.0��Ŀ����ʹFe3+��Zn2+��ȫ��������ȥ�����㳣����Zn��OH��2���ܶȻ�����Ksp[Zn��OH��2]=1.0��10-17��
��2��д����Һ2�е�Mn2+���MnO2�����ӷ���ʽMn2++H2O2+2OH-=MnO2��+2H2O��
��3��д������B��ϡ������pH=5ʱ��Ӧ�Ļ�ѧ����ʽZn��OH��2+H2SO4=ZnSO4+2H2O��
��4�������л����Խ���Һ4��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ����ᾧˮ�������ξ��壮
��5��MnO2��������ȡKMnO4����һ�������½�MnO2����ΪK2MnO4��Ȼ�������������������������K2MnO4��Һ�õ�KMnO4�����K2MnO4��Һ���ܷ�Ӧ����ʽΪ2K2MnO4+2H2O$\frac{\underline{\;���\;}}{\;}$2KMnO4+2KOH+H2����

��֪�����̷ۡ�����Ҫ�ɷ���MnO2��Zn��OH��2��MnOOH��̼�ۣ��������������κ������Σ������£������������������pH���±���
| ���� | Fe��OH��3 | Fe��OH��2 | Zn��OH��2 | Mn��OH��2/Mn��OH��3 |
| ��ʼ����pH | 2.7 | 7.6 | 5.7 | 8.3 |
| ��ȫ����pH ��c��1.0��10-5mol/L�� | 3.7 | 9.6 | 8.0 | 8.8 |
��2��д����Һ2�е�Mn2+���MnO2�����ӷ���ʽMn2++H2O2+2OH-=MnO2��+2H2O��
��3��д������B��ϡ������pH=5ʱ��Ӧ�Ļ�ѧ����ʽZn��OH��2+H2SO4=ZnSO4+2H2O��
��4�������л����Խ���Һ4��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ����ᾧˮ�������ξ��壮
��5��MnO2��������ȡKMnO4����һ�������½�MnO2����ΪK2MnO4��Ȼ�������������������������K2MnO4��Һ�õ�KMnO4�����K2MnO4��Һ���ܷ�Ӧ����ʽΪ2K2MnO4+2H2O$\frac{\underline{\;���\;}}{\;}$2KMnO4+2KOH+H2����
17����һ��֬������ͨ��һϵ�з�Ӧ�ɱ�Ϊ�����������ִ�Ҳ��ͨ����ȥ���������������Ӿ۷�Ӧ�ȱ仯���ת��Ϊһ�ָ߾���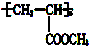 �����ִ��Ľṹ��ʽΪ��������
�����ִ��Ľṹ��ʽΪ��������
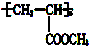 �����ִ��Ľṹ��ʽΪ��������
�����ִ��Ľṹ��ʽΪ��������| A�� | CH2�TCHCH2OH | B�� | CH3CH2OH | C�� | CH3CH��OH��CH2OH | D�� | CH2ClCHClCH2OH |
4��ijԭ���װ����ͼ��ʾ������ܷ�ӦΪ2Ag+Cl2=2AgCl������˵����ȷ���ǣ�������


| A�� | ������ӦΪAg-e-=Ag+ | |
| B�� | �ŵ�ʱ������Ĥ�����Һ���д�����ɫ�������� | |
| C�� | ����·��ת��0.1mole-ʱ��ͨ������Ĥ��������Ϊ0.2mol | |
| D�� | ��KCl��Һ�������ᣬ�����ܷ�Ӧ��ı� |
2�����л�̬ԭ�ӻ����ӵĵ����Ų�ʽ������ǣ�������
| A�� | K 1s22s22p63s23p64s1 | B�� | Mg2+ 1s22s22p6 | ||
| C�� | F- 1s22s22p5 | D�� | Br 1s22s22p63s23p63d104s24p5 |