��Ŀ����
9��ij�о�С�������������о�
��1����ͬѧ�����A��B��Cһ��ʵ�飨��ͼ1��ʾ����̽��������������������ϳ�ʱ���ͬѧ�۲쵽�������ǣ�A���������⣻B�����������⣬C�����������⣮
��ͨ������ʵ������������ɵó���������ⲿ�����ǺͿ����Լ�ˮ�Ӵ���
��A�����������绯��ʴ�������缫��ӦʽΪO2+2H2O+4e-=4OH-��
��ʵ��B���õ�ˮҪ������д�����ֲ���͵������Ǹ��������е�������
��ʵ��C�м�ʯ�ҵ����������տ����е�ˮ������
��2����ͼ2��ʾ���Թ��з�һ����������������ij�������Һ�У������۲죬���ݹ۲쵽�IJ�ͬ����ش��������⣺
�ٹ���Һ������½����ұ����������Թ��еĵ������Һ������Ϊ���ԣ������ĵ缫��ӦʽΪ��4H++4e-�T2H2����
�ڵ�����Һ���ұ��½���������������Թ������������ĵ绯��ʴΪ������ʴ�������ĵ缫��ӦʽΪ��Fe-2e-�TFe2+��
��3����ͼ3��ʾ��������ʢ�к�ˮ���������и�ʴʱ�ɿ쵽����˳���ǣ�4������2������1������3����
���� ��1�������ڳ�ʪ�Ŀ����������绯ѧ��ʴ�������������ڸ���Ŀ����������γ�ԭ��ط�Ӧ��
���������绯ѧ��ʴʱ�������������������õ��ӵĻ�ԭ��Ӧ��
�۽�ˮ��п��Խ�ˮ�еĿ����ų���ֲ���ͺ�ˮ�ǻ������ܵģ�
�ܼ�ʯ���ǿ�����ˮ�ģ�
��2�������к���̼������̼�ͺ��ʵĵ������Һ����ԭ��أ��������Ի����������£�������������ʴ����������ʧ���ӷ���������Ӧ�������������õ��Ӻ�ˮ��Ӧ�������������ӣ������������£����������ⸯʴ����������ʧ���ӷ���������Ӧ�������������ӵõ��ӷ�����ԭ��Ӧ��
��3����������ʴ����˳��Ϊ����������������ԭ��ظ�������ѧ��ʴ����ԭ����������������������ݴ˷������
��� �⣺��1������������ⲿ�����ǽ���Ҫ�Ϳ����е�ˮ�Լ������Ӵ����ʴ�Ϊ���Ϳ����Լ�ˮ�Ӵ���
�����������绯��ʴ����������Ϊ���ý�������ʧȥ���Ӷ��������������������������õ��ӵķ�ӦO2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��ˮ���ܽ���һ���Ŀ�������п��Խ������ų���ֲ���ͺ�ˮ�ǻ������ܵģ����������Ǹ��������е��������ʴ�Ϊ����У����������е�������
�ܼ�ʯ������ˮ���������������տ����е�ˮ�������ʴ�Ϊ�����տ����е�ˮ������
��2�������к���̼������̼�ͺ��ʵĵ������Һ����ԭ��أ�
�ٹ���Һ������½����ұ�������˵�������ĸ�ʴ���������嵼��ѹǿ��������������������ⸯʴ����Һ�����ԣ���������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Fe-2e-�TFe2+�������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��4H++4e-�T2H2�����ʴ�Ϊ�����ԣ�4H++4e-�T2H2����
�ڵ�����Һ���ұ��½������������˵������ʻ����������Ի������ԣ��Թ����������ˮ��Ӧ��ʹ��ѹǿ��С����������������ʴ��������������������ʧ���ӷ���������Ӧ���缫��ӦʽΪ��Fe-2e-�TFe2+�������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H2O+O2+4e-�T4OH-��
�ʴ�Ϊ��������ʴ��Fe-2e-�TFe2+��
��3����������ʴ����˳��Ϊ����������������ԭ��ظ�������ѧ��ʴ����ԭ�����������������������1������������ѧ��ʴ����2����Fe�����Դ���Sn����ԭ��ظ�������3����Fe�Ļ��С��Zn����ԭ�����������4����Fe����������������Fe��ʴ����˳���ǣ�4������2������1������3�����ʴ�Ϊ����4������2������1������3����
���� ���⿼������ĵ绯ѧ��ʴ�Լ�������ע�����������¸����������ⸯʴ�������Ի����������¸�������������ʴ����ȷ������ʴ����˳���ǽⱾ��ؼ���֪��ԭ��غ͵���װ�����������缫���Ƽ��ɽ����Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 16 | B�� | 14 | C�� | 12 | D�� | 28 |
| A�� | CH4+2O2$\stackrel{��ȼ}{��}$CO2+2H2O | |
| B�� | H2C=CH2+Br2��CH3CHBr | |
| C�� |  | |
| D�� | CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3CH2CH3+H2O |
 ����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ�
����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ�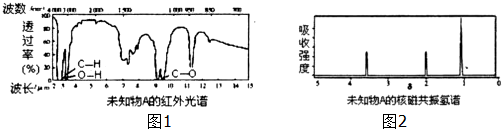
 ��1���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�� ���壮ij�ϳɰ���ҵ����������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��CH4��g��+2H2O ��g��?CO2��g��+4H2��g����Ӧ�����������仯��ͼ��ʾ����÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ���
��1���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�� ���壮ij�ϳɰ���ҵ����������Ȼ����ˮ��Ӧ�Ʊ�������Ҫ��ӦΪ��CH4��g��+2H2O ��g��?CO2��g��+4H2��g����Ӧ�����������仯��ͼ��ʾ����÷�ӦΪ���ȷ�Ӧ������ȡ����ȡ��� =CHCOOCH2CH3����·�����£�
=CHCOOCH2CH3����·�����£�


 ���뻯����DCH3COOCH2CH3������Ӧ����ֱ�Ӻϳ��л���Q����D�Ľṹ��ʽΪCH3COOCH2CH3��������C����Ϊ����ȩ��
���뻯����DCH3COOCH2CH3������Ӧ����ֱ�Ӻϳ��л���Q����D�Ľṹ��ʽΪCH3COOCH2CH3��������C����Ϊ����ȩ��