��Ŀ����
4�� ����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ�
����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28��C��һ�����壬E��һ�ָ߷��ӻ����A��B��C��D��E��һ�������´�����ͼת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���1��A��B�ķ�Ӧ����ʽΪCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��2��Bͬ���칹��Ľṹ��ʽΪCH3OCH3��D��ͬϵ������Է���������С�����ʵĽṹ��ʽΪCH4��
��3����Ӧ�١��������ڼӳɷ�Ӧ���ǣ�����ţ��ڢܣ�
���� ����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28����AΪCH2=CH2����ϩˮ�����ɵ�BΪ�Ҵ�CH3CH2OH���Ҵ���Na��Ӧ���ɵ�����C������H2����ϩ�������ڴ����������·����ӳɷ�Ӧ���ɵ�DΪ����CH3CH3����ϩ��һ�������·����Ӿ۷�Ӧ���ɵĸ߷��ӻ�����EΪ����ϩ���ݴ˷�����
��� �⣺����A��ʯ���ѽ����Ҫ����֮һ������Է�������Ϊ28����AΪCH2=CH2����ϩˮ�����ɵ�BΪ�Ҵ�CH3CH2OH���Ҵ���Na��Ӧ���ɵ�����C������H2����ϩ�������ڴ����������·����ӳɷ�Ӧ���ɵ�DΪ����CH3CH3����ϩ��һ�������·����Ӿ۷�Ӧ���ɵĸ߷��ӻ�����EΪ����ϩ��
��1��AΪCH2=CH2����ϩˮ�����ɵ�BΪ�Ҵ�CH3CH2OH����Ӧ����ʽΪCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��2���Ҵ���ͬ���칹��ֻ�м��ѣ����ѵĽṹ��ʽΪCH3OCH3��DΪ����CH3CH3��D��ͬϵ������Է���������С�����ʵĽṹ��ʽΪCH4���ʴ�Ϊ��CH3OCH3��CH4��
��3����ͼ��Ӧ�Т��ǼӾ۷�Ӧ�����Ǽӳɷ�Ӧ������ȡ����Ӧ�����Ǽӳɷ�Ӧ���ʴ�Ϊ���ڢܣ�
���� ���⿼���л����ƶϣ���Ҫ������ϩ���Ļ�ѧ���ʣ��ѶȲ���ע�����֪ʶ���������գ�

| A�� | H2O��g��=H2��g��+$\frac{1}{2}$ O2��g����H=+242 kJ/mol | B�� | 2 H2��g��+O2��g��=2 H2O��g����H=-484 kJ/mol | ||
| C�� | H2��g��+$\frac{1}{2}$ O2��g��=H2O��g����H=+242 kJ/mol | D�� | 2 H2��g��+O2��g��=2 H2O��g����H=+484 kJ/mol |

| A�� | ������̬���ĺ����ǽ�����������Ҫָ�꣬��Щ��������ͨ�����еĵ����ʷ����ֽⷴӦ���ɵ� | |
| B�� | �����嵥�еı����������ڷ����� | |
| C�� | С���е���ҪӪ����������ά�� | |
| D�� | ����ǿ�����͡��ɰ���Ԥ��ȱ����ƶѪ�����������ò�����Ҳ�����������ж� |
| A�� | ����������ˮ��Ӧ��2Na2O2+2H2O�T4Na++4OH-+O2�� | |
| B�� | ������������Һ�к�������Һ��Ba2++OH-+H++SO42-�TH2O+BaSO4�� | |
| C�� | ��������ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| D�� | �ڳ���ʯ��ˮ��ͨ�������CO2��Ca2++2OH-+2CO2�TCa��HCO3��2 |
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04mol
��3�������ݼ�����BaCl2��Һ�ø������6.27g������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A�� | K+��һ������ | B�� | 100 mL��Һ�к�0.01 mol CO32- | ||
| C�� | Cl-���ܴ��� | D�� | Ba2+һ�������ڣ�Mg2+���ܴ��� |



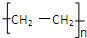 ��
�� CH3COOC2H5+H2O����Ӧ���ͣ�������Ӧ��
CH3COOC2H5+H2O����Ӧ���ͣ�������Ӧ��