äãá¢áÖàï
È´14ñøÈˋQÀÂWÀÂXÀÂYÀÂZƒªöˆåˆùÄøÉóÖÝÚøÅú¯ùáøÉóÖåˆùÄȘúØóðåÙæÆÅ·ò»ØâÇöå—ǵȘQåˆùÄçáî¶âŠæƤùëãößçÓæÆȘWåˆùÄåÙæÆçáæŸëãýÐçÓæÆò»òúóðÇöëãýÐçÓæÆò»çá2ÝÑȘYòúçÄ¢úøŤ˜ê¢æŸÑÁçáåˆùÄȘZáÉÅö°è¤šè¨È´£·æˋ¤šè¨ÈˋçáZ2O¤ë¤Öè¨çáZOê§øøî¾£₤öÿÀÈ
ÂéWö£ÆÖåˆùÄøÉóÖÝÚçÖ øÉóÖçÖ æÍÀÈYçáçÖØ£çÓâŠáÉ È´äŸÀ¯ÇµÆÖÀÝ£·À¯ÅÀÆÖÀÝÈˋXçáçÖØ£çÓâŠáÉÀÈ
ÂóXQ3ñøæÆøÅçá£₤îÏ¥■âÁÅëöˆ È´äŸÀ¯¥¨ÅåÀÝ£·À¯ñú¥¨ÅåÀÝÈˋ¿ý¥Ü¥■Ș¢í¥ðâÁÅëöˆ ÀÈQÀˆXˋpQÀˆYˋpQÀˆWøÅ¥■°ÊæŸÑäçáòú ÀÈ
ÂúZçᣪä˜åÙæƤùëãçÓæÆééý¥ò§òú ȘZçáçËøòÆŠXçá柡ԥÜî¾£₤öÿÑåÆÎùÛ£₤öÿçáüÀàÉؤñÇÆÎçáâŠæÆñ§°äò§öˆ ÀÈ
ÂàØîøˆÈ¤ÂìWQ4(g) Ȩ4XY2(g) ˋ4XY(g)ȨWY2 (g)Ȩ2Q2Y(g) À¼HˋÈÙ574KJÀÊmol-1
ÂÖWQ4(g) Ȩ4XY(g) ˋ2X2 (g) ȨWY2 (g) Ȩ2Q2Y(g) À¼HˋÈÙ1160KJÀÊmol-1
å·ÆèWQ4£¿åÙXY2躰èX2çáàà£₤îÏñ§°äò§öˆ ÀÈ
È´1ÈˋѱÀ¶AȘÅÀÆÖȣȴ2Èˋ¥¨ÅåÀÂ໧úæÑÅöÀÂH-OÈ£
È´3Èˋ1s22s23s23p63d104s1ÀÂ3Cu+8H++2NO3-=3Cu2++2NOÀ■+2H2OÈ£
È´4ÈˋCH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ÎÊH=ÈÙ867KJ/molÈ£
§ãö—òåäãñøö—Ȥ¡ªƒïäãØã¢èøˆí㥡øøåˆùÄñøÝÞòúȤQòúHÈ£WòúCÈ£XòúNÈ£YòúOÈ£ZòúCuÀÈÂéCåˆùÄåÖåˆùÄøÉóÖÝÚøÅö£ÆÖçÖѱøÉóÖçÖ¶AÈ£ÆèÆÖNåˆùÄçáåÙæÆçáæŸëãýÐçáçÓæÆÇÎÆÖ¡û¿šçâçᯊ°ðôºçáöàÑ´æÇä˜È˜òÏàËçÓæƧüáîȘùªØåNçáçÖØ£çÓâŠáÉǵÆÖOçáçÖØ£çÓâŠáÉÀÈÂóåÖNH3ñøæÆøÅçá£₤îÏ¥■âÁÅëöˆ¥ŸÅå¿ý¥Ü¥■Ș¡ûñøæÆçá¢í¥ðâÁÅëöˆà»§úæÑÅöÈ£ÆèÆÖCÀÂNÀÂOà»øøåˆùÄçáåÙæƯŠƒÑøާ˥¾ÅÀȘùªØåCÀˆHÈ£NÀˆHÈ£OÀˆHçá¥■°Êå§âÇå§ÑäȘ¥■áɃëå§âÇå§ÇµÈ˜Ø·Çù¥■°ÊæŸÑäçáOÀˆH¥■È£ÂúåÙæÆÇÎÆÖਰðôºÀ¯Š°ðôº£·à¨¢íòÝòúöàÑ´çáæÇä˜È˜Ø·Çù29¤éåˆùÄCuçᣪä˜åÙæƤùëãçÓæÆééý¥ò§òú1s22s23s23p63d104s1; CuçáçËøòÆŠNçá柡ԥÜî¾£₤öÿÑåÆÎùÛ£₤öÿHNO3çáüÀàÉؤñÇÆÎçáâŠæÆñ§°äò§öˆ3Cu+8H++2NO3-=3Cu2++2NOÀ■+2H2Oȣȴ4Èˋ(Âì+ÂÖ)Àô2í«âÚ¢èçûCH4(g)+2NO2(g)=N2(g)+CO2(g)+2H2O(g) ÎÊH=ÈÙ867KJ/molÈ£
¢¥çÐȤ¢¥ýÕåˆùÄçáëóÑüÀÂöÿøòñøæÆøÛ¥ð£₤îÏ¥■ÀÂñøæÆçá¢í¥ð¿¿ÅëÀÂåÙæÆçáçÓæÆééý¥ò§ÀÂåˆùÄçáçÓâŠáÉçáÝà§üÀÂâŠæÆñ§°äò§ÀÂàà£₤îÏñ§°äò§çáòÕÅÇçáøˆòÑÀÈ

 ¢ÖùÐä㢴¥ÆÆÎÆûäã¥₤îçüçêÅÇÞ¯¡
¢ÖùÐä㢴¥ÆÆÎÆûäã¥₤îçüçêÅÇÞ¯¡ æܤüæåýãüçêÅÇÞ¯¡
æܤüæåýãüçêÅÇÞ¯¡aÀÂbÀÂcÀÂdÀÂeòúçÖà»øÉóÖçáöÍøøåˆùÄȘa¤ëbçá柡ԥÜî¾£₤öÿçáùÛ£₤öÿü奟ÅåȘúØ¥ŸÅåbȃaȘc¤ëdçáó½ä˜úã£₤öÿçáöàÑ´ÅåcȃdȘeçáçËøò¥àáÉÆŠúãî¾£₤áóàÉؤñÇÆÎȘØýáÉÆŠüÀê·ùÃñÇÆÎȘýÂúØÑ¥ýºèºúãó½È˜å·ù■ûúçáåÙæÆÅ·ò»ÆèÅÀç§Çµçáù°Å·òú
| AÈÛaÀÂbÀÂeÀÂdÀÂc | BÈÛbÀÂaÀÂcÀÂeÀÂd |
| CÈÛbÀÂaÀÂeÀÂdÀÂc | DÈÛbÀÂaÀÂdÀÂcÀÂe |
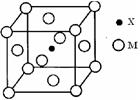
á¢ú¯È˜Ø§êóèüò¿ÆûñéèðÅå¤ùùÄ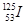 øöêóøæê—Ș¡û¤ùùÄåÙæƤùáÖçáøÅæÆò»ÆŠçÓæÆò»øÛýŸòú
øöêóøæê—Ș¡û¤ùùÄåÙæƤùáÖçáøÅæÆò»ÆŠçÓæÆò»øÛýŸòú
| AÈÛ 125 | BÈÛ72 | CÈÛ19 | DÈÛ53 |
È´12ñøÈˋÆÅAÀÂBÀÂCÀÂDÀÂEöÍøøåÙæÆÅ·ò»ÅÀÆÖ18çáåˆùÄȘóðäÄí¼ÅéüÂàÓüôȤ
| åˆùÄÝÁ¤é | äÄí¼Åéü |
| A | óðøÅØ£øøåÙæƤùáÖø£ÆÅøòæÆû£ÆÅøÅæÆ |
| B | óðåÙæÆçáLýÐçÓæÆò»òúKýÐçá3ÝÑ |
| C | óðî¶âŠæÆÆŠBçáؾâŠæƃÔÆÅüÁë˜çáçÓæÆýЧÿ¿È˜ úؤùçÓ¤èò»ÆŠBüÁýŸ3 |
| D | óðåÙæÆçáæŸëãýÐçÓæÆò»çàÆÖçÓæÆýÐò»È˜úØòúçÄ¢ú øŤ˜ê¢§üÑÁçáåˆùÄøÛØ£ |
| E | çËøòöˆ£óôäè¨ó½äÍȘ¢èÆûÆÖøóåšó₤¯æñÜ |
È´1ÈˋÆèAÀÂBÀÂCà»øøåˆùÄÅö°èçá£₤¤üöÿ¤˜ÆÅçá£₤îÏ¥■âÁÅëòú ÀÈ
È´2ÈˋÆèAÀÂBÀÂCåˆùÄøÅê§ê§æÕ¤üÅö°èçá£₤¤üöÿüÁ£ËñÇÆÎ躰èçËøòçá£₤îÏñ§°ä
ò§ ÀÈ
È´3ÈˋDçËøòÆŠÆèAÀÂBÀÂCà»øøåˆùÄÅö°èçá£₤¤üöÿçáùÛàÉؤñÇÆÎçáâŠæÆñ§°äò§
ÀÈ
È´4ÈˋÅÇ°—òçîÕòØÆûàÚû䢵ȴMnO2ÈˋøóàÀEçá£₤îÏñ§°äò§ ÀÈ
È´5ÈˋC¤ëDê§åˆùÄÅö°èçáçËøò£ŸóûÅˋ§üú¢çáòú È´ÅÇåˆùÄñ«¤éÈˋȘéÅÑüØâƒïòú ÀÈ
È´10ñøÈˋüôÝÚòúåˆùÄøÉóÖÝÚçáØ£ý¢ñø, íŠÑåÝÚøÅçáÂìÀ¨ÂÃøøåˆùÄ,äŸÅÇüôêÅ¢í¯æ:
| ø¼æÍ øÉóÖ | ÂþA | ·A | µA | ¶A | ¾A | —A | ¼A | 0æÍ |
| 2 | | | | Âì | ÂÖ | ÂÜ | | |
| 3 | ÂÉ | | Âï | | | Âß | ÂÔ | ÂÁ |
| 4 | ÂÃ | | | | | | | |
È´2ÈˋåÖ柡ԥÜî¾£₤öÿçáùÛ£₤öÿøÅȘùÃÅåæŸú¢çá£₤¤üöÿçáñøæÆò§òú_______Ș¥ŸÅåæŸú¢çá£₤¤üöÿçáçÓæÆò§òú:_____________ÀÈ
È´3Èˋ柡ԥÜî¾£₤öÿòúê§Ååî¾£₤öÿçáåˆùÄòú_____________È£ÅÇ°—ù■çáî¾£₤öÿÆŠúãî¾£₤áóñÇÆÎçáâŠæÆñ§°äò§____________________________________________ÀÈ
È´4ÈˋÆûçÓæÆò§ÝÚòƒåˆùÄÂÉÆŠÂßÅö°èçáçá£₤¤üöÿçáÅö°è¿»°ä_________________________ Ș¡û£₤¤üöÿò¶ÆÖ__________________(äŸ À¯¿ý¥ÜÀÝ£·À¯âŠæÆÀÝ)£₤¤üöÿÀÈ
È´5ÈˋåˆùÄÂìÆŠÂÔÅö°èçáçá£₤¤üöÿçáçÓæÆò§öˆ______________________ Ș¡û£₤¤üöÿòúÆè___________ È´äŸÀ¯¥¨ÅåÀÝÀ¯ñú¥¨ÅåÀÝÈˋ¥■Åö°èçáÀÈ
ÀÂüôë¥òúåˆùÄøÉóÖÝÚçáØ£ý¢ñøȘóðøÅû¢¡—ò»æøÝÁ¤éǺÝÚÑåÆÎçáØ£øøåˆùÄÀÈ
| Âì | | | ||||||
| | | | | ÂÖ | ÂÜ | ÂÉ | | |
| Âï | | Âß | ÂÔ | | | ÂÁ | ÂÃ | |
È´1Èˋ£Ù°—åˆùÄÂÖçáåÙæƧÿ¿òƒØãë¥ ÀÈ
È´2Èˋò¶ÆÖüÀÆÅó½äÍçáåˆùÄÝÁ¤éòú_______Ș¢è漯Šç¥äÍýáêüçáåˆùÄÝÁ¤éòú______ÀÈ
È´3ÈˋÂÜÀÂÂÔê§øøåˆùÄüÁÝà§üȘñú§Þò¶Ååú¢çáòú È´äŸåˆùÄñ«¤éÈˋÀÈ
È´4ÈˋåˆùÄÂÉçáçËøòÆŠåˆùÄÂïçáçËøòñÇÆ΢è躰èê§øø£₤¤üöÿȘ£₤îÏò§ñøÝÞòú Ș ÀÈ