��Ŀ����
��12�֣���A��B��C��D��E����ԭ������С��18��Ԫ�أ���������Ϣ���£�
| Ԫ�ر�� | ������Ϣ |
| A | ����һ��ԭ�Ӻ���ֻ������û������ |
| B | ��ԭ�ӵ�L���������K���3�� |
| C | ����������B�������Ӿ�����ͬ�ĵ��Ӳ�ṹ�� �Һ˵������B���3 |
| D | ��ԭ�ӵ��������������ڵ��Ӳ��������ǵؿ� �к����϶��Ԫ��֮һ |
| E | ����Ϊ����ɫ���壬����������Ư�� |
��1����A��B��C����Ԫ���γɵĻ����ﺬ�еĻ�ѧ�������� ��
��2����A��B��CԪ������������γɵĻ��������Ӧ���ɵ��ʵĻ�ѧ����
ʽ ��
��3��D��������A��B��C����Ԫ���γɵĻ������ˮ��Һ��Ӧ�����ӷ���ʽ
��
��4��д��ʵ���������̿�MnO2����ȡE�Ļ�ѧ����ʽ ��
��5��C��D��Ԫ���γɵĵ��ʻ���Щ��ǿ���� ��дԪ�ط��ţ����ж������� ��
��ÿ��2�֣�
�����Ӽ��������ԣ����ۼ�����2Na2O2+2H2O=4NaOH+O2��(NaH+H2O=NaOH+H2��)
��2Al+2OH-+2H2O=2AlO2-+3H2������MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��Na �����ƿ��Ժ���ˮ���ҷ�Ӧ �������ԣ�����������Ҳ�ɣ���
�������������A��һ��ԭ�Ӻ���ֻ������û�����ӣ���AΪHԪ�أ�Bԭ�ӵ�L���������K���3������BΪOԪ�أ�C��������B�������Ӿ�����ͬ�ĵ��Ӳ�ṹ���Һ˵������B���3����CΪNaԪ�أ�Dԭ�ӵ��������������ڵ��Ӳ��������ǵؿ��к����϶��Ԫ��֮һ����DΪAlԪ�أ�E����Ϊ����ɫ���壬����������Ư�ۣ���EΪClԪ�ء�
��1����A��B��C����Ԫ���γɵĻ�����ΪNaOH�����еĻ�ѧ�������ǣ����Ӽ��������ԣ����ۼ���
��2����A��B��CԪ������������γɵĻ��������Ӧ���ɵ��ʵķ�ӦΪ��Na2O2��H2O��Ӧ����NaOH��O2����ѧ����ʽΪ��2Na2O2+2H2O=4NaOH+O2������NaH��H2O��Ӧ����NaOH��H2����ѧ����ʽΪ��NaH+H2O=NaOH+H2����
��3��D����ΪAl��A��B��C����Ԫ���γɵĻ�����ΪNaOH��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
��4��MnO2��Ũ���ᷴӦ����MnCl2��Cl2��H2O����ѧ����ʽΪ��MnO2+4HCl(Ũ) MnCl2+Cl2��+2H2O��
MnCl2+Cl2��+2H2O��
��5��CԪ���γɵĵ���Ϊ����Na��DԪ���γɵĵ���Ϊ����Al�������ƿ��Ժ���ˮ���ҷ�Ӧ �������ԣ������Խ�ǿ�ĵ�����Na��
���㣺���⿼��Ԫ�ص��ƶϡ����ʵ��������Ʊ�������ʽ����д����ѧ�����жϡ�

����ͬλ�أ�����˵������ȷ����
| A����������ͬ����������ͬ����ѧ���ʼ�����ͬ |
| B����������ͬ����������ͬ����ѧ������ͬ |
| C����������ͬ����������ͬ����ѧ���ʼ�����ͬ |
| D����������ͬ����������ͬ����ѧ���ʲ�ͬ |
�۵���������ԭ����������ͬ�ķ��ӡ����ӻ�ԭ���ų�Ϊ�ȵ����壬�ȵ�����������ƵĽṹ�����ʡ�����ѡ���л���Ϊ�ȵ��������
A��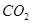 �� �� 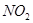 | B��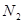 �� ��  |
C��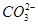 �� �� 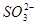 | D�� �� �� 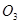 |
��֪A��B��C��D��E��F��Gλ��Ԫ�����ڱ���ǰ�����ڣ���Ԫ��ԭ�������������ӣ�A��ɫ��Ӧ�ʻ�ɫ����ҵ���õ��B�����ڵ��Ȼ������Ʊ�B��C��һ���ܱ�HF��NaOH��Һ�ܽ�ĵ��ʣ�D�ĵ縺�Ա��״�һ������ȴ����С��E�������Ʊ�Ư��Һ��ԭ�ϣ�F���γɺ�ɫ(��ש��ɫ)�ͺ�ɫ�����������G��һ�����������
��1��ǰ����������Ԫ���У���̬ԭ����δ�ɶԵ�������������������ͬ��Ԫ���� �֡�
��2��Ԫ��A��B��C�ֱ�����������γ�����X��Y��Z�۵���±���
| ������ | X | Y | Z |
| �۵�/K | 1266 | 1534 | 183 |
��3����֪���������£����Է���DOE2��һ��Һ̬���������ԭ��D���ӻ���ʽ�� ����ʢ��10mLˮ����ƿ�еμ�������DOE2��Һ�����������д̼�����ζ�����塣����д�˷�Ӧ�Ļ�ѧ����ʽ ��
��4��G�뵪ԭ�ӿ�1��1���ϣ��γ��˹��ϳɵ����Ͱ뵼����ϣ��侧��ṹ�뵥�������ơ�Gԭ�ӵļ۵����Ų�ʽΪ ���ڸúϳɲ����У���ͬһ��Gԭ��������Nԭ�ӹ��ɵĿռ乹��Ϊ�������塣�����ֻ������������У��˾������� ���塣
��5��F����Ķѻ���ʽ�� (��ѻ�����)������λ��Ϊ �� ��F����������Һ�еμӰ�ˮֱ��������д���˹������漰���������ӷ���ʽ ���ݼ۲���ӶԻ������ۣ�Ԥ��SO42-�Ŀռ乹��Ϊ ��