��Ŀ����
20�����жԼ������������������ȫ����ȷ������ǣ�����������Kͨ��������ú���У��Ը���������ĽӴ�
�ڴ�Li��Cs���������ˮ��ӦԽ��Խ������
���Ƶ���ɫ��Ӧ�ʻ�ɫ����ɫ��Ӧ�ǻ�ѧ�仯
�ܼ���������ӣ���������ǿ����Li+
�ݼ������ԭ�Ӱ뾶�����Ӱ뾶����˵���������������
��Li��Cs����������ܶ�Խ��Խ���ۡ��е�Խ��Խ��
��CsOH��ǿ����Ա�KOHǿ
��Cs2CO3����ʱ�ֽ��CO2��Cs2O��
| A�� | �ڢۢޢ� | B�� | �ڢۢܢ� | C�� | �ܢݢޢ� | D�� | �٢ܢݢ� |
���� �ټص��ܶȴ���ú�ͣ��Ϳ�����ˮ������������������̼������Ӧ��
�ڴ�Li��Cs����������ǿ���������ˮ��ӦԽ��Խ���ң�
���Ƶ���ɫ��Ӧ�ʻ�ɫ����ɫ��Ӧ�������仯
�ܽ�����Խǿ����Ӧ�����ӵ�������Խ����
�ݵ��Ӳ���Խ�࣬�뾶Խ�����Լ������ԭ�Ӱ뾶�����Ӱ뾶����˵���������������
��Li��Cs����������ܶ�һ��Խ��Խ��ط������۷е㽵�ͣ�
�߽�����Խǿ����������Ӧ��ˮ����ļ���Խǿ��
��������̼���ε��ȶ������ӣ�
��� �⣺�ټص��ܶȴ���ú�ͣ��Ϳ�����ˮ������������������̼������Ӧ������Kͨ��������ú���У��Ը���������ĽӴ����ʢ���ȷ��
�ڴ�Li��Cs����������ǿ���������ˮ��ӦԽ��Խ���ң��ʢڴ���
����ɫ��Ӧ�������仯���ʢ۴���
�ܽ�����Խǿ����Ӧ�����ӵ�������Խ�������Լ���������ӣ���������ǿ����Li+���ʢ���ȷ��
�ݵ��Ӳ���Խ�࣬�뾶Խ�����Լ������ԭ�Ӱ뾶�����Ӱ뾶����˵��������������ʢ���ȷ��
��Li��Cs����������ܶ�һ��Խ��Խ��ط��������ϵ����۷е㽵�ͣ��ʢ���
�߽�����Խǿ����������Ӧ��ˮ����ļ���Խǿ������CsOH��ǿ����Ա�KOHǿ���ʢ���ȷ��
��������̼���ε��ȶ������ӣ�����Cs2CO3����ʱ���ֽ��CO2��Cs2O���ʢ����
��ѡA��
���� ���⿼���˼�������ʵݱ���ɵķ����жϣ�ע���������ʷ��������������ʵ��жϣ����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�| A�� | ��������ǿ���ԣ�������ʴ�̲��� | |
| B�� | ̼���л�ԭ�ԣ�һ���������ܽ��������軹ԭΪ�� | |
| C�� | BaCO3��BaSO4��������ˮ��������ҽ���ϵı��� | |
| D�� | ���������кܸߵ��۵㣬���������������ռ������ |
| A�� | ��λʱ��������n mo1 O2��ͬʱ����2n mol NO2 | |
| B�� | ����������ɫ���ٸı� | |
| C�� | ��������ѹǿ���ٸı��״̬ | |
| D�� | ���������ܶȲ��ٸı��״̬ |
| A�� | �պ��ľ̿���ȵ�Ũ���� | B�� | ͭ��Ũ���Ṳ�� | ||
| C�� | ʳ�κ�Ũ���Ṳ�� | D�� | �����е���Ũ���� |
| A�� | CH4��C2H4��C3H6��������� | |
| B�� | CH4��C3H6��C2H2����C3H6��C2H2=1��2�����ʵ���֮�ȣ� | |
| C�� | C2H6��C4H6��C2H2ͬ�����������Ϊ2��1��2 | |
| D�� | C3H8��C4H8��C2H2������Ϊ11��14��26 |
| ���� | ���� |
| ��ʢ��4g��Na2O2���ձ��м���50mL����ˮ�õ���Һa | ���з�Ӧ��������ʹ������ľ����ȼ������ |
| ȡ5mL��Һa���Թ��У��������η�̪ | ������Һ��� ����10���ֺ���Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
��2��������Һ��ɫ��������Һa�д��ڽ϶��H2O2���̪�����˷�Ӧ��
��ͬѧͨ��ʵ��֤ʵ��H2O2�Ĵ��ڣ�ȡ������Һa�������Լ�MnO2���ѧʽ���������������
����ͬѧ�������ϻ�Ϥ����KMnO4���ԲⶨH2O2�ĺ�����ȡ15.00mL��Һa����ϡH2SO4�ữ����μ���
0.003mol•L-1��KMnO4��Һ���������壬��Һ��ɫ���ʿ�ʼ�������죬���յ�ʱ������20.00mL��KMnO4��Һ��
������ƽ��2MnO4-+5H2O2+6H+�T2Mn2++5O2��+8H2O
����Һa��c��H2O2��=0.01mol•L-1��
����Һ��ɫ���ʿ�ʼ���������ԭ������Ƿ�Ӧ���ɵ�Mn2+��������
��3��Ϊ̽����������ԭ��ͬѧ�Ǽ�������������ʵ�飺
����H2O2��Һ�е������η�̪��������5��0.1mol•L-1 NaOH��Һ����Һ�����Ѹ�ٱ���ɫ�Ҳ������壬10���Ӻ���Һ����ɫ��
������0.1mol•L-1 NaOH��Һ�е������η�̪�ģ�����Һ��죬10���Ӻ���Һ��ɫ�����Ա仯�������Һ��ͨ��O2����Һ��ɫ�����Ա仯��
�ٴ�ʵ���͢��У��ɵó��Ľ����Ǽ��������£�H2O2�����̪��Ӧ��O2���ܣ�
��ͬѧ�ǽ�һ��ͨ��ʵ��֤ʵ����Һa�е����̪��H2O2���̪�����˻�ѧ��Ӧ��ʵ�鷽���ǣ�ȡ������Һa���Թ��У�����MnO2����ַ�Ӧ�����ϲ���Һ�е���2�η�̪���죬10���Ӻ���Һ��ɫ�����Ա仯��
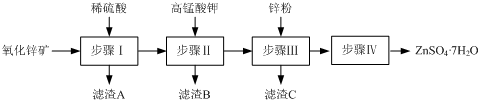
 ��
�� ���ṹʽΪH-O-H��
���ṹʽΪH-O-H��