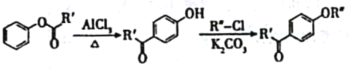
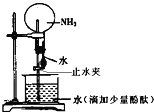
��Ŀ����
����Ŀ�����������һ�ָ�Ч��ܵ����ͷ�����ɫ����������Ҫ������ˮ������ʵ��С���Ʊ�������أ�K2FeO4����̽�������ʡ�
���ϣ�K2FeO4Ϊ��ɫ���壬����KOH��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���O2���ڼ�����Һ�н��ȶ���
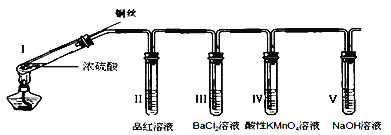
(1)�Ʊ�K2FeO4���г�װ���ԣ�
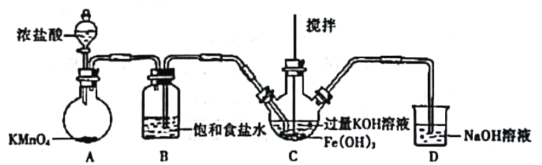
����ͼ��ʾ��AΪ��������װ�á�װ��A��B��C��D�д��ڴ������______________������ţ���
��C�еõ���ɫ�������Һ��C��ͨ������������Ӧ�����ɸ�����أ�K2FeO4���Ļ�ѧ����ʽΪ_______������Cl2�������������KOH��Ӧ��
(2)̽��K2FeO4������
��ȡC����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���Cl2��Ϊ֤���Ƿ�K2FeO4��������Cl��������Cl2��������·�����
����I | ȡ������Һa���μ�KSCN��Һ����������Һ�ʺ�ɫ�� |
����II | ��KOH��Һ���ϴ��C�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һb��ȡ����b���μ����ᣬ��Cl2������ |
i.�ɷ���I����Һ����֪��Һa�к���__________���ӣ��������ӵĴ��ڲ����ж�һ����K2FeO4��������Cl2����ΪK2FeO4����������Һ�в��ȶ�����д��K2FeO4��������Һ�з�����Ӧ�����ӷ���ʽ___________________________________��
ii���������֤��K2FeO4��������Cl������KOH��Һϴ�ӵ�Ŀ����_______________��
�ڸ���K2FeO4���Ʊ�ʵ��ó���������Cl2_____FeO42�� �����������������������ʵ�������Cl2��FeO42����������ǿ����ϵǡ���෴��ԭ����_______________��
(3)�����Ʊ�װ��C�м���Fe(OH)3������Ϊ14.0g����ַ�Ӧ���ˡ�ϴ�ӡ������K2FeO4����19.3g����K2FeO4�IJ���Ϊ______________��
���𰸡�B 3C12+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O Fe3+ 4FeO42-+20H+=4Fe3++3O2��+10H2O ȷ��K2FeO4�ڼ��Ի����е��ȶ��ԣ�ͬʱ�ų�ClO-����֤�ĸ��� > ��Һ������Բ�ͬ 74.5%
��������
��1����AΪ��������װ�ã�B��ȥ�����е�HCl��Ӧ�����ܣ��Ҳ�̵��ܣ������̳���װ��A��B��C��D�д��ڴ������B������ţ����ʴ�Ϊ��B��
��C�еõ���ɫ�������Һ��C��ͨ���������������������������ɸ�����أ�K2FeO4������ѧ����ʽΪ3C12+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O���ʴ�Ϊ��3C12+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O��
��2����i.Fe3+������SCN-������Ϸ�Ӧ�����ɺ�ɫ�������ӣ��ɷ���I����Һ����֪��Һa�к���Fe3+���ӣ��������ӵĴ��ڲ����ж�һ����K2FeO4��������Cl2����ΪK2FeO4��������Һ�в��ȶ���K2FeO4��������Һ�з�����Ӧ�����ӷ���ʽ4FeO42-+20H+=4Fe3++3O2��+10H2O���ʴ�Ϊ��Fe3+��4FeO42-+20H+=4Fe3++3O2��+10H2O��
ii���������֤��K2FeO4��������Cl������KOH��Һϴ�ӵ�Ŀ����ȷ��K2FeO4�ڼ��Ի����е��ȶ��ԣ�ͬʱ�ų�ClO-����֤�ĸ��š��ʴ�Ϊ��ȷ��K2FeO4�ڼ��Ի����е��ȶ��ԣ�ͬʱ�ų�ClO-����֤�ĸ��ţ�
�ڸ���K2FeO4���Ʊ�ʵ��ó���3C12+2Fe(OH)3+10KOH=2K2FeO4+6KCl+8H2O����������������ǿ���������������Cl2��FeO42�� ����������ʵ�������Cl2��FeO42����������ǿ����ϵǡ���෴��ԭ������Һ������Բ�ͬ���ʴ�Ϊ��������Һ������Բ�ͬ��
��3�������Ʊ�װ��C�м���Fe(OH)3������Ϊ14.0g����ַ�Ӧ���ˡ�ϴ�ӡ������K2FeO4����19.3g��������ԭ���غ㣺
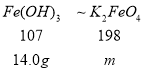
m=![]() =25.9g����K2FeO4�IJ���Ϊ
=25.9g����K2FeO4�IJ���Ϊ![]() =74.5%��
=74.5%��
�ʴ�Ϊ��74.5%��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�