��Ŀ����
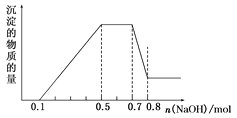
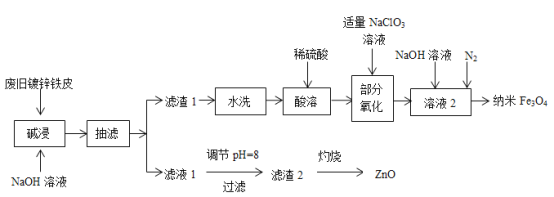
����Ŀ��¶���ڿ����е�ij�������ƹ�����Ʒ����ˮ����������Һ����μ���ϡ���������������ɵ�CO2����״������������������Ĺ�ϵ��ͼ��ʾ��������CO2��ˮ�е��ܽ⣩��
��1��д�� 0��150mL �η�����Ӧ�����ӷ���ʽΪ��____________��150mL ��200mL�η�����Ӧ�����ӷ���ʽΪ��___________________��
��2��������������ʵ���Ũ��Ϊ_______________��
��3���������������ƹ�����Ʒ����ˮ�����γ���Һ������Ϊ___________��_________��д��ѧʽ���������ʵ����ֱ�Ϊ ____________��_______________��
���𰸡�OH��+H+=H2O��CO32��+H��=HCO3�� HCO3-+H+ =CO2+H2O 0.4 molL-1 NaOH Na2CO3 0.04 mol 0.02 mol
��������
(1)��Ϊ̼���������ᷴӦ����̼�����ƣ���̼�����������ᷴӦ���ɶ�����̼������������ʵ�����ȣ�������ͼ��֪V(HCl)��0��100mL�����ڷ�����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O����100��150mL�����ڷ�����Ӧ�Ļ�ѧ����ʽΪNa2CO3+HCl=NaCl+NaHCO3����150��200mL�����ڷ�����Ӧ�Ļ�ѧ����ʽΪNaHCO3+HCl=NaCl+H2O+CO2����
(2)����150mL��200mL���ᣬ������Ӧ��NaHCO3+HCl�TNaCl+CO2��+H2O���������ɶ�����̼����n(HCl)���ٸ���c=![]() ���㣻
���㣻
(3)����200mL����ʱ������0.448L������̼����ʱ��Һ������ΪNaCl�����������غ����n(NaCl)������̼Ԫ���غ����n(Na2CO3)�������������غ����n(NaOH)���ݴ˽��
(1)��Ϊ̼���������ᷴӦ����̼�����ƣ���̼�����������ᷴӦ���ɶ�����̼������������ʵ�����ȣ�����ͼ��֪V(HCl)��0��100mL�����ڷ�����Ӧ�����ӷ���ʽΪ��OH��+H+=H2O����100��150mL�����ڷ�����Ӧ�����ӷ���ʽΪCO32��+H��=HCO3������150��200mL�����ڷ�����Ӧ�Ļ�ѧ����ʽΪHCO3-+H+ =CO2��+H2O��
(2)����150mL��200mL���ᣬ�����������=200mL-150mL=50mL������0.448L������̼�������ʵ���=![]() =0.02mol�����NaHCO3 +HCl�TNaCl+CO2��+H2O��֪�μӷ�Ӧ��HCl�����ʵ���Ϊ0.02mol����������Һ�����ʵ���Ũ��Ϊ
=0.02mol�����NaHCO3 +HCl�TNaCl+CO2��+H2O��֪�μӷ�Ӧ��HCl�����ʵ���Ϊ0.02mol����������Һ�����ʵ���Ũ��Ϊ![]() =0.4mol/L��
=0.4mol/L��
(3)��(1)�Ľ�����֪�������ƹ�����Ʒ�к���NaOH��Na2CO3���������Ʒ����ˮ�����γ���Һ������ΪNaOH��Na2CO3������200mL����ʱ������0.448L������̼����ʱ��Һ������ΪNaCl�����������غ��֪n(NaCl)=0.2L��0.4mol/L=0.08mol�������̼Ԫ���غ�n(Na2CO3)=n(CO2)=0.02mol�������������غ�n(NaOH)=n(NaCl)-2n(Na2CO3)=0.08mol-0.02mol��2=0.04mol��

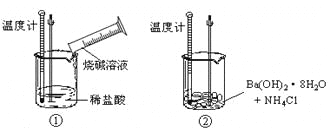
����Ŀ��Ϊ�ﵽʵ��Ŀ�ģ�����ѡ�õ�װ�á�ʵ���������ȷ����
ʵ��Ŀ�� | ʵ�鲽���װ�� | |
A | �Ƚ�H2O2��Fe3+�������� | �������ữ��˫��ˮ����Fe(NO3)2��Һ�� |
B | ����100 mL 1.0 mol��L��1NaOH��Һ | ��100mL����ƿ�м���4.0 g NaOH���壬��ˮ���̶��� |
C | ��֤�������������������� |
�����缫�����μ����軯����Һ |
D | ̽���¶ȶԷ�Ӧ���ʵ�Ӱ�� | ����Ƭ��С��ͬ�ĵ��۵⻯����ֽ�ֱ��������֧�����ͬ���Թ��У�����ֽ�ϵμ�2.0 mol��L��1��H2SO4��Һ2��3�Σ��ܷ��Թܣ��ֱ������º�40��ˮԡ�з�Ӧ���۲첢��¼��ɫʱ�� |
A.AB.BC.CD.D
����Ŀ�����������һ�ָ�Ч��ܵ����ͷ�����ɫ����������Ҫ������ˮ������ʵ��С���Ʊ�������أ�K2FeO4����̽�������ʡ�
���ϣ�K2FeO4Ϊ��ɫ���壬����KOH��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���O2���ڼ�����Һ�н��ȶ���
(1)�Ʊ�K2FeO4���г�װ���ԣ�
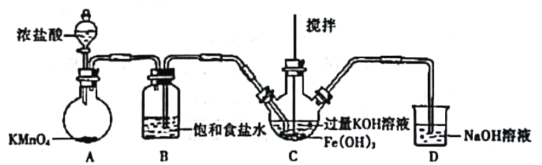
����ͼ��ʾ��AΪ��������װ�á�װ��A��B��C��D�д��ڴ������______________������ţ���
��C�еõ���ɫ�������Һ��C��ͨ������������Ӧ�����ɸ�����أ�K2FeO4���Ļ�ѧ����ʽΪ_______������Cl2�������������KOH��Ӧ��
(2)̽��K2FeO4������
��ȡC����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���Cl2��Ϊ֤���Ƿ�K2FeO4��������Cl��������Cl2��������·�����
����I | ȡ������Һa���μ�KSCN��Һ����������Һ�ʺ�ɫ�� |
����II | ��KOH��Һ���ϴ��C�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һb��ȡ����b���μ����ᣬ��Cl2������ |
i.�ɷ���I����Һ����֪��Һa�к���__________���ӣ��������ӵĴ��ڲ����ж�һ����K2FeO4��������Cl2����ΪK2FeO4����������Һ�в��ȶ�����д��K2FeO4��������Һ�з�����Ӧ�����ӷ���ʽ___________________________________��
ii���������֤��K2FeO4��������Cl������KOH��Һϴ�ӵ�Ŀ����_______________��
�ڸ���K2FeO4���Ʊ�ʵ��ó���������Cl2_____FeO42�� �����������������������ʵ�������Cl2��FeO42����������ǿ����ϵǡ���෴��ԭ����_______________��
(3)�����Ʊ�װ��C�м���Fe(OH)3������Ϊ14.0g����ַ�Ӧ���ˡ�ϴ�ӡ������K2FeO4����19.3g����K2FeO4�IJ���Ϊ______________��