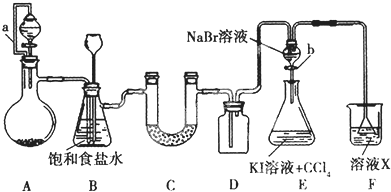
��Ŀ����
4����������Ҫ�Ļ�����Ʒ֮һ����1���ϳɰ��õ����������Լ���Ϊԭ���Ƶã��йػ�ѧ��Ӧ�������仯��ͼ�ס�����ʾ����Ӧ���Ƿ��ȷ�Ӧ������ȡ������ȡ������ж������Ƿ�Ӧ����������������������������CH4��g����H2O��g����Ӧ����CO��g����H2 ��g�����Ȼ�ѧ����ʽ��CH4��g��+H2O��g��=CO��g��+3H2 ��g����H=+161.1kJ•mol-1��
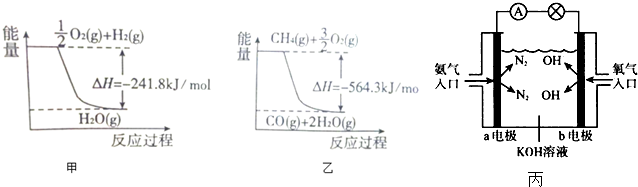
��2��ͼ���ǰ���ȼ�ϵ�ص�ʾ��ͼ���ش��������⣺
��a�缫�������������������������������������缫��ӦʽΪ2NH3-6e-+6OH-=N2+6H2O��
�ڷ�Ӧһ��ʱ��������Һ��pH����С���������С�����䡱����
�����ø�ȼ�ϵ�ز����ĵ�������Ƥ�϶�п���ư���Ƥ����ij��Ƥ������Ҫ���� 97.5gп��������������Ҫ���İ���22.4 L����״������
���� ��1������ͼ�������Ӧ����������������������������˵����Ӧ�Ƿ��ȷ�Ӧ��д����Ӧ�Ȼ�ѧ����ʽ�����ݸ�˹���ɼ���õ������Ȼ�ѧ����ʽ��
��2���ٰ���ȼ�ϵ�أ�ȼ�ϰ����ڸ����Ϸŵ磬��ϼ��Ի���д��������Ӧ��
�ڸ����ܷ�Ӧ�жϵ������Һ��pH�仯��
�۸���ԭ��صĸ�����ʧȥ�ĵ��ӵ����ʵ������ڵ����������ϵõ��ĵ��ӵ����ʵ������ݴ˷�����
��� �⣺��1��ͼ�������Ӧ����������������������������˵����Ӧ�Ƿ��ȷ�Ӧ����Ӧ���Ȼ�ѧ����ʽΪ��
��H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8KJ/mol
��CH4��g��+$\frac{3}{2}$O2��g��=CO��g��+2H2O��g����H=-564.3KJ/mol
�ɸ�˹���ɢ�-�١�3�õ���CH4��g��+H2O��g��=CO��g��+3H2 ��g����H=-564.3+241.8��3=+161.1kJ•mol-1��
�ʴ�Ϊ�����ȣ���Ӧ����������������������������CH4��g��+H2O��g��=CO��g��+3H2 ��g����H=+161.1kJ•mol-1��
��2���ٰ���ȼ�ϵ�أ�ȼ�ϰ����ڸ�����ʧ���ӷ���������Ӧ����ӦΪ2NH3-6e-+6OH-=N2+6H2O������a�缫���������ʴ�Ϊ������2NH3-6e-+6OH-=N2+6H2O��
�������������Ϸŵ磬���ڵ�����Ǽ��Եģ���������ӦΪ��O2+2H2O+4e-=4OH-�������ܷ�ӦΪ4NH3+3O2=2N2+6H2O������ˮ�����Լ�������Ӧһ��ʱ��������Һ��pH����С���ʴ�Ϊ����С��
��ij��Ƥ������Ҫ����9.75gп�����ʵ���n=$\frac{m}{M}$=$\frac{97.5}{65}$=1.5mol���������ϵ�1.5mol��2=3mol���ӣ�
ԭ��صĸ�����ʧȥ�ĵ��ӵ����ʵ������ڵ����������ϵõ��ĵ��ӵ����ʵ��������ܷ�Ӧ4NH3+3O2=2N2+6H2O��������ӦΪ2NH3+6OH--6e-=N2+6H2O����֪1molNH3ʧȥ3mol���ӣ���Ҫʧȥ3mol���ӣ�����Ҫ�İ��������ʵ���Ϊ1mol���ڱ���µ����Ϊ22.4L���ʴ�Ϊ��22.4��
���� ���⿼���˸�˹���ɵ�Ӧ�ú͵缫��Ӧ����д����Ŀ�ۺ��Խϴ��ѣ��Ƕ�֪ʶ���ۺ����á�ע�����֪ʶ���������գ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �������ƹ������Ȼ�粒���ķ�Ӧ | B�� | �������Ȼ��⻯�� | ||
| C�� | �����������ķ�Ӧ | D�� | ʯ��ʯ�ķֽⷴӦ |
| A�� | ��λ��Ԫ�����ڱ��ĵ������ڵڢ��� | |
| B�� | ����ͨ��װ�г�ɫ�������ظ���ص�װ�ü��˾���Ƿ�ƺ�ݳ� | |
| C�� | ��֪��Ԫ�ص�һ��ͬλ�ص�������Ϊ53�����ͬλ����53������ | |
| D�� | ������Ϊ29�ĸ�ԭ�ӷ��ű�ʾΪ${\;}_{24}^{29}$Cr |
| A�� | Na2O2 | B�� | Al��OH��3 | C�� | H2SO4 | D�� | BaSO4 |
Ϊ�������к��е����ӣ���������ʵ�飺
���ò�����պȡԭ��Һ����pH��ֽ�ϣ���ֽ�Ժ�ɫ��
����ȡ����ԭ��Һ����BaCl2��Һ�����ɲ�����ϡ����İ�ɫ������
��ȡ�����ϲ���Һ�����ữ����������Һ�����ɰ�ɫ������
���й���ԭ��Һ��˵����ȷ���ǣ�������
| A�� | ԭ��Һ��һ��������Ba2+��HCO3- | |
| B�� | ȡ������Һ����KSCN����Һ�Ժ�ɫ����ԭ��Һһ����Fe3+ | |
| C�� | ԭ��Һ��һ������SO42-��Cl- | |
| D�� | Ϊȷ��ԭ��Һ���Ƿ���K+����ͨ����ɫ��Ӧֱ�ӹ۲���ɫ�Ƿ�Ϊ��ɫ��ȷ�� |
| A�� | �����и�ԭ������㶼����8�����ȶ��ṹ | |
| B�� | ���Ǿ�Ϊ60�㣬���Ǻ��м��Լ��ļ��Է��� | |
| C�� | BF3�е����BCl3�е㣬��ΪB-F�������� | |
| D�� | BF3�����ȶ��Ը���BCl3����ΪB-F�����ܸ� |
| A�� | ȡ������ҺX�������м�������������ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2+ | |
| B�� | ��1 mL 1%��NaOH��Һ�м���2 mL 2%��CuSO4��Һ�����ټ���0.5 mL�л���X�����Ⱥ�δ����ש��ɫ������˵��X�в�����ȩ�� | |
| C�� | ��CuSO4��Һ�м���KI��Һ���а�ɫ�������ɣ��ټ������Ȼ�̼�����Ȼ�̼�����ɫ��˵����ɫ��������ΪCuI | |
| D�� | ��Ũ�Ⱦ�Ϊ0.1 mol•L-1��MgCl2��CuCl2�����Һ����μ��백ˮ������������ɫ������˵��Ksp[Cu��OH��2]��Ksp[Mg��OH��2] |
| ʵ���� | HA | NaOH | ��Ϻ���Һ��pH |
| �� | C��HA��=0.2mol•L-1 | C��NaOH��=0.2mol•L-1 | pH=a |
| �� | C��HA��=c1mol•L-1 | C��NaOH��=0.2mol•L-1 | pH=7 |
| �� | C��HA��=0.1mol•L-1 | C��NaOH��=0.1mol•L-1 | pH=9 |
| �� | pH=2 | pH=12 | pH=b |
��1���������������ʵ���������Ӽ�����������������a�������Һ��pH����˵��HA��ǿ�ỹ�������a=7����HA��ǿ���a��7����HA�����ᣮ
��2���������������ʵ��������c1�������������������=����0.2mol•L-1������ʵ����HA��NaOH��Һ���ǰ��HA��Һ��C��A-����NaOH��Һ��C��Na+���Ĺ�ϵ��B
A��ǰ�ߴ� B�����ߴ� C��������� D�����жϣ�
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����c��Na+����c��A-����c��OH-����c��H+�������У�C��A-��=0.1+1��10-9-1��10-5mol•L-1�����������Ƽ��㣬�ش�ȷֵ�������һ��Ҫ����
��4������ʵ���У�HA��NaOH��Һ���ǰ��C��HA���������������������=����C��NaOH����b��7�����������������=����