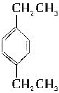
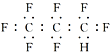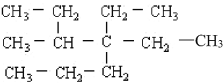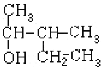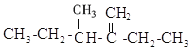МвДїДЪИЭ
ЎѕМвДїЎїСх»ЇСЗµЄ(N2O)КЗТ»ЦЦЗїОВКТЖшМеЈ¬ЗТТЧЧЄ»»іЙїЕБЈОЫИѕОпЎЈСРѕїСх»ЇСЗµЄ·ЦЅв¶Ф»·ѕі±Ј»¤УРЦШТЄТвТеЎЈ
ЈЁ1Ј©ОЫЛ®ЙъОпНСµЄ№эіМЦРЈ¬ФЪТмСшОўЙъОпґЯ»ЇПВЈ¬ПхЛбп§їЙ·ЦЅвОЄN2OєНБнТ»ЦЦІъОпЈ¬ёГ·ґУ¦µД»ЇС§·ЅіМКЅОЄ___ЎЈ
ЈЁ2Ј©ТСЦЄ·ґУ¦2N2O(g)=2N2(g)+O2(g)µДЎчH=-163kJ/molЈ¬1molN2(g)Ј¬1molO2(g)·ЦЧУЦР»ЇС§јь¶ПБСК±·Ц±рРиТЄОьКХ945kJЈ¬498kJµДДЬБїЈ¬Фт1molN2O(g)·ЦЧУЦР»ЇС§јь¶ПБСК±РиТЄОьКХµДДЬБїОЄ___kJЎЈ
ЈЁ3Ј©ФЪТ»¶ЁОВ¶ИПВµДєгИЭИЭЖчЦРЈ¬·ґУ¦2N2O(g)=2N2(g)+O2(g)µДІї·ЦКµСйКэѕЭИзПВЈє
·ґУ¦К±јд/min | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
cЈЁN2OЈ©/molL-1 | 0.100 | 0.090 | 0.080 | 0.070 | 0.060 | 0.050 | 0.040 | 0.030 | 0.020 | 0.010 | 0.010 |
ўЩФЪ0Ў«20minК±¶ОЈ¬·ґУ¦ЛЩВКvЈЁN2OЈ©ОЄ___molL-1min1ЎЈ
ўЪИфN2OЖрКјЕЁ¶Иc0ОЄ0.150 molL-1Ј¬Фт·ґУ¦ЦБ30minК±N2OµДЧЄ»ЇВК¦Б=___ЎЈ±ИЅПІ»Н¬ЖрКјЕЁ¶ИК±N2OµД·ЦЅвЛЩВКЈєvЈЁc0=0.150molL-1Ј©___vЈЁc0=0.001molL-1Ј©ЈЁМоЎ°ЈѕЎ±ЎўЎ°=Ў±»тЎ°ЈјЎ±Ј©ЎЈ
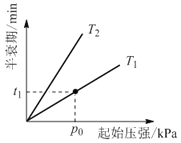
ўЫІ»Н¬ОВ¶ИЈЁTЈ©ПВЈ¬N2O·ЦЅв°лЛҐЖЪЛжЖрКјС№ЗїµД±д»Ї№ШПµИзНјЛщКѕЈЁНјЦР°лЛҐЖЪЦёИОТ»ЕЁ¶ИN2OПыєДТ»°лК±ЛщРиµДПаУ¦К±јдЈ©Ј¬ФтT1____T2ЈЁМоЎ°ЈѕЎ±ЎўЎ°=Ў±»тЎ°ЈјЎ±Ј©ЎЈµ±ОВ¶ИОЄT1ЎўЖрКјС№ЗїОЄp0Ј¬·ґУ¦ЦБt1minК±Ј¬МеПµС№Зїp=___ЈЁУГp0±нКѕЈ©ЎЈ
ЈЁ4Ј©µвХфЖшґжФЪДЬґу·щ¶ИМбёЯN2OµД·ЦЅвЛЩВКЈ¬·ґУ¦АъіМОЄЈє
µЪТ»ІЅ I2(g) ![]() 2I(g) ЈЁїм·ґУ¦Ј©
2I(g) ЈЁїм·ґУ¦Ј©
µЪ¶юІЅ I(g)+N2O(g)ЎъN2(g)+IO(g) ЈЁВэ·ґУ¦Ј©
µЪИэІЅ IO(g)+N2O(g)ЎъN2(g)+O2(g)+I(g) ЈЁїм·ґУ¦Ј©
КµСй±нГчЈ¬є¬µвК±N2O·ЦЅвЛЩВК·ЅіМv=kcЈЁN2OЈ©[cЈЁI2Ј©]0.5ЈЁkОЄЛЩВКіЈКэЈ©ЎЈПВБР±нКцХэИ·µДКЗ___ЈЁМо±кєЕЈ©ЎЈ
A.N2O·ЦЅв·ґУ¦ЦРЈ¬k(є¬µв)Јѕk(ОЮµв)
B.µЪТ»ІЅ¶ФЧЬ·ґУ¦ЛЩВКЖрѕц¶ЁЧчУГ
C.µЪ¶юІЅ»о»ЇДЬ±ИµЪИэІЅґу
D.I2ЕЁ¶ИУлN2O·ЦЅвЛЩВКОЮ№Ш
Ўѕґр°ёЎїNH4NO3![]() N2OЎь+2H2O 1112.5 1ЎБ10-3 20% ЈЅ Јѕ 1.25p0 AC
N2OЎь+2H2O 1112.5 1ЎБ10-3 20% ЈЅ Јѕ 1.25p0 AC
ЎѕЅвОцЎї
ЈЁ1Ј©ФЪТмСшОўЙъОпґЯ»ЇПВЈ¬ПхЛб淋ЄФЄЛШјЫМ¬№йЦРїЙ·ЦЅвОЄN2OєНЛ®Ј¬ѕЭґЛКйРґЈ»
ЈЁ2Ј©ёщѕЭ·ґУ¦ИИ=·ґУ¦ОпµДЧЬјьДЬ-ЙъіЙОпµДЧЬјьДЬЅвµГЈ»
ЈЁ3Ј©ўЩёщѕЭv=![]() јЖЛгЈ»
јЖЛгЈ»
ўЪ№ЫІмКэѕЭїЙµГГїёф10minЈ¬c(N2O)µД±д»ЇБїПаµИЈ¬№КµҐО»К±јдДЪc(N2O)µД±д»ЇБїКЗ¶ЁЦµЈ¬јґN2OµД·ЦЅвЛЩВККЗ¶ЁЦµЈ¬јґОЄ1ЎБ10-3molL-1min-1Ј¬ѕЭґЛїЙµГЈ»
ўЫОВ¶ИФЅёЯ·ґУ¦ЛЩВКФЅїмЈ»ёщѕЭ·ґУ¦їЙЦЄЈ¬2molN2O·ґУ¦Т»°лК±ИЭЖчЦРЖшМеЧЬ№І2.5molЈ¬ёщѕЭПаН¬ОВ¶ИПВЈ¬ЖшМеµДОпЦКµДБїУлС№ЗїіЙХэ±ИїЙµГЈ»
ЈЁ4Ј©AЈ®УЙМвµвµДґжФЪМбёЯN2OµД·ЦЅвЛЩВКЈ¬v=kcЈЁN2OЈ©[cЈЁI2Ј©]0.5ЦРvУлkіЙХэ±ИЈ»
BЈ®Вэ·ґУ¦¶ФЧЬ·ґУ¦ЛЩВКЖрѕц¶ЁЧчУГЈ»
CЈ®µЪ¶юІЅ·ґУ¦ВэЈ¬»о»ЇДЬґуЈ»
DЈ®I2ЕЁ¶ИУлN2O·ЦЅвЛЩВКУР№ШЎЈ
ЈЁ1Ј©ПхЛб淋ЄФЄЛШјЫМ¬№йЦРЈ¬їЙ·ЦЅвОЄN2OєНЛ®Ј¬»ЇС§·ґУ¦ОЄЈєNH4NO3![]() N2OЎь+2H2OЈ»
N2OЎь+2H2OЈ»
ЈЁ2Ј©Йи1molN2O(g)·ЦЧУЦР»ЇС§јь¶ПБСК±РиТЄОьКХµДДЬБїОЄQЈ¬ФтЎчH=2Q-2ЎБ945kJ/mol-498kJ/mol=-163kJmol-1Ј¬ЅвµГQ=1112.5kJ/molЈ»
ЈЁ3Ј©ўЩФЪ0Ў«20minК±¶ОЈ¬·ґУ¦ЛЩВКv(N2O)=![]() =
=![]() =1ЎБ10-3molL-1min-1Ј»
=1ЎБ10-3molL-1min-1Ј»
ўЪ№ЫІмКэѕЭїЙµГГїёф10minЈ¬c(N2O)µД±д»ЇБїПаµИЈ¬№КµҐО»К±јдДЪc(N2O)µД±д»ЇБїКЗ¶ЁЦµЈ¬јґN2OµД·ЦЅвЛЩВККЗ¶ЁЦµЈ¬јґОЄvЈЁc0=0.150molL-1Ј©=vЈЁc0=0.100molL-1Ј©=1ЎБ10-3molL-1min-1Ј¬ИфN2OЖрКјЕЁ¶Иc0ОЄ0.150molL-1Ј¬Фт·ґУ¦ЦБ30minК±N2OµДЧЄ»ЇВКОЄ![]() ЎБ100%=20%Ј»
ЎБ100%=20%Ј»
ўЫОВ¶ИФЅёЯ·ґУ¦ЛЩВКФЅїмЈ¬УЙНјїЙЦЄЈ¬T1МхјюПВЛщУГК±јдЅП¶МЈ¬·ґУ¦ЛЩВКїмЈ¬ФтT1ЈѕT2Ј»ёщѕЭ·ґУ¦їЙЦЄЈ¬2molN2O·ґУ¦Т»°лК±ИЭЖчЦРЖшМеЧЬ№І2.5molЈ¬Фт![]() =
=![]() Ј¬ЅвµГp=1.25p0Ј»
Ј¬ЅвµГp=1.25p0Ј»
ЈЁ4Ј©AЈ®УЙМвµвµДґжФЪМбёЯN2OµД·ЦЅвЛЩВКЈ¬v=kcЈЁN2OЈ©[cЈЁI2Ј©]0.5ЦРvУл
BЈ®Вэ·ґУ¦¶ФЧЬ·ґУ¦ЛЩВКЖрѕц¶ЁЧчУГЈ¬µЪ¶юІЅЖрѕц¶ЁЧчУГЈ¬№КBґнОуЈ»
CЈ®µЪ¶юІЅ·ґУ¦ВэЈ¬»о»ЇДܴ󣬹КCХэИ·Ј»
DЈ®ёщѕЭN2O·ЦЅвЛЩВК·ЅіМv=kcЈЁN2OЈ©[cЈЁI2Ј©]0.5Ј¬I2ЕЁ¶ИУлN2O·ЦЅвЛЩВКУР№ШЈ¬№КDґнОуЈ»
№Кґр°ёОЄЈєACЎЈ

 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ёЎѕМвДїЎїПЦУРІї·ЦФЄЛШµДРФЦКУлФЧУ![]() »т·ЦЧУ
»т·ЦЧУ![]() Ѕб№№Из±нЛщКѕЈє
Ѕб№№Из±нЛщКѕЈє
ФЄЛШ±аєЕ | ФЄЛШРФЦКУлФЧУ |
T | ЧоНвІгµзЧУКэКЗґОНвІгµзЧУКэµД3±¶ |
X | іЈОВПВµҐЦК·ЦЧУОЄЛ«ФЧУ·ЦЧУЈ¬·ЦЧУЦРє¬УР3¶Ф№ІУГµзЧУ¶Ф |
Y | MІг±ИKІгЙЩ1ёцµзЧУ |
Z | µЪИэЦЬЖЪФЄЛШµДЅрКфАлЧУЦР°лѕ¶ЧоРЎ |
ЈЁ1Ј©»іцФЄЛШTµДФЧУЅб№№КѕТвНјЈє__ЎЈ
ЈЁ2Ј©ФЄЛШYУлФЄЛШZПа±ИЈ¬ЅрКфРФЅПЗїµДКЗ__![]() УГФЄЛШ·ыєЕ±нКѕ
УГФЄЛШ·ыєЕ±нКѕ![]() Ј¬ПВБР±нКцЦРДЬЦ¤ГчХвТ»КВКµµДКЗ___
Ј¬ПВБР±нКцЦРДЬЦ¤ГчХвТ»КВКµµДКЗ___![]() МоЧЦДё
МоЧЦДё![]() ЎЈ
ЎЈ
a.YµҐЦКµДИЫµг±ИZµҐЦКµН
b.YµД»ЇєПјЫ±ИZµН
c.YµҐЦКУлЛ®·ґУ¦±ИZµҐЦКУлЛ®·ґУ¦ѕзБТ
d.YЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпµДјоРФ±ИZµДЗї
ЈЁ3Ј©TЎўXЎўYЎўZЦРµДБЅЦЦФЄЛШДЬРОіЙјИУРАлЧУјьУЦУР·Зј«РФ№ІјЫјьµД»ЇєПОпЈ¬РґіцёГ»ЇєПОпµД»ЇС§КЅЈє__ЎЈ
ЈЁ4Ј©TїЙТФРОіЙОИ¶ЁµДТхАлЧУTm-Ј¬YїЙТФРОіЙОИ¶ЁµДСфАлЧУYn+Ј¬Жд°лѕ¶№ШПµОЄr(Tm-)__r(Yn+)(МоЎ°>Ў±Ј¬Ў°<Ў±»тЎ°=Ў±)
ЈЁ5Ј©XЎўYЎўZµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпїЙТФП໥·ўЙъ·ґУ¦Ј¬РґіцYЎўZµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпЦ®јд·ґУ¦µДАлЧУ·ЅіМКЅ___ЎЈ