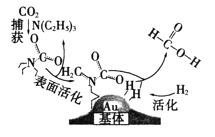
��Ŀ����
����Ŀ����������3���Ȼ�ѧ����ʽ��
H2(g)+![]() O2(g)=H2O(g)����H1= -a kJ��mol-1��
O2(g)=H2O(g)����H1= -a kJ��mol-1��
H2(g)+![]() O2(g)=H2O(l)����H2= -b kJ��mol-1��
O2(g)=H2O(l)����H2= -b kJ��mol-1��
2H2(g)+O2(g)=2H2O(l)����H3= -c kJ��mol-1��
�����±����е�ԭ�����Ƶ���������۵���(����)
ѡ�� | ԭ�� | ���� |
A | H2��ȼ���Ƿ��ȷ�Ӧ | a��b��c�������� |
B | �ٺ͢������ʵĻ�ѧ����������ͬ | a=b |
C | �ٺ͢���H2O��״̬��ͬ����ѧ��������ͬ | a��c�������κι�ϵ |
D | �۵Ļ�ѧ�������Ǣڵ�2�� | ��H2����H3 |
A.AB.BC.CD.D
���𰸡�A
��������
A. ����ȼ�յĹ����Ƿ��ȹ��̣������ʱ�С��0����a��b��c�������㣬��A��ȷ��
B. �ʱ�Ĵ�С�뷴Ӧ����������״̬�йأ�����ʽ�١���������ˮ��״̬��һ�£���a������b����B����
C. �۵�ϵ���Ǣڵ�2�������Ԣ۵��ʱ�Ϊ�ڵ�2��������Ϊ��̬ˮת��ΪҺ̬ˮ�Ĺ���Ϊ���ȹ��̣����Ԣڵ��ʱ�С�ڢٵ��ʱ䣬������a��b��c��Ϊ���������2b=c>2a����C����
D. �۵�ϵ���Ǣڵ�2�������Ԣ۵��ʱ�Ϊ�ڵ�2������c=2b����Ϊ����ȼ���Ƿ��ȹ��̣�������H��0������H2>��H3����D����
��ȷ����A��

��ϰ��ϵ�д�
�����Ŀ