��Ŀ����
10��Ԫ�����ڱ���һ���֣��ش��������⣨��Ԫ�ط�����д����| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | VIIA | O |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | ��10�� | ��11�� | ��12�� |
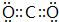
��2��д�����ԭ�ӽṹʾ��ͼ
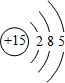 ��
����3������ЩԪ���У�����õĽ���Ԫ�ص�����������Ӧˮ����ĵ���ʽΪ
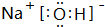 ���л�ѧ��Ϊ���Ӽ������ۼ�
���л�ѧ��Ϊ���Ӽ������ۼ���4����ЩԪ�ص����������Ķ�Ӧˮ������HClO4������ǿ���γ�������������Ļ�ѧʽ��Al��OH��3���������߷�Ӧ�����ӷ���ʽAl��OH��3+3H+�TAl3++3H2O
��5���Ӣݵ���11����Ԫ���У�Clԭ�Ӱ뾶��С��
���� ��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪN����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪP����ΪS����ΪCl����ΪAr��
��1���١�������Ԫ���γɵĸ�̬������ΪCO2��������Cԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�
��2��PԪ��ԭ�Ӻ�����15�����ӣ���3�����Ӳ㣬���������Ϊ2��8��5��
��3������Ԫ���У�����õĽ���Ԫ�ص�����������Ӧˮ����ΪNaOH��
��4����Ԫ��û����ۺ����ᣬ�ʸ������������ǿ����������������������������߷����кͷ�Ӧ����Al��ClO4��3��H2O��
��5��ͬ�����������ԭ�Ӱ뾶��С��
��� �⣺��Ԫ�������ڱ���λ�ã���֪��ΪC����ΪN����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪP����ΪS����ΪCl����ΪAr��
��1���١�������Ԫ���γɵĸ�̬������ΪCO2��������Cԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ������ʽΪ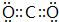 ���ʴ�Ϊ��
���ʴ�Ϊ��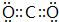 ��
��
��2��PԪ��ԭ�Ӻ�����15�����ӣ���3�����Ӳ㣬���������Ϊ2��8��5��ԭ�ӽṹʾ��ͼΪ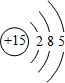 ���ʴ�Ϊ��
���ʴ�Ϊ��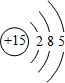 ��
��
��3������Ԫ���У�����õĽ���Ԫ�ص�����������Ӧˮ����ΪNaOH���������������������ӹ��ɣ�����ʽΪ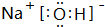 ���������Ӽ������ۼ����ʴ�Ϊ��
���������Ӽ������ۼ����ʴ�Ϊ��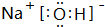 �����Ӽ������ۼ���
�����Ӽ������ۼ���
��4����Ԫ��û����ۺ����ᣬ��HClO4��������ǿ��Al��OH��3������������������߷����кͷ�Ӧ����Al��ClO4��3��H2O����Ӧ���ӷ���ʽΪ��Al��OH��3+3H+�TAl3++3H2O���ʴ�Ϊ��HClO4��Al��OH��3��Al��OH��3+3H+�TAl3++3H2O��
��5��ͬ�����������ԭ�Ӱ뾶��С���ʴӢݵ���11����Ԫ����Clԭ�Ӱ뾶��С���ʴ�Ϊ��Cl��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɣ��ѶȲ���ע���Ԫ�����ڱ������գ������ڻ���֪ʶ�Ĺ��̣�

| A�� | ��״���£�11.2LSO3������ԭ����Ϊ1.5NA | |
| B�� | ���³�ѹ�£�1.8g H2O�к��еĵ�����Ϊ0.8NA | |
| C�� | ���³�ѹ�£�48g O2��O3�Ļ�����к��е���ԭ����Ϊ3NA | |
| D�� | ��״���£�0.1mol Cl2������NaOH��Һ��Ӧʱ��ת�Ƶĵ�����Ϊ0.2NA |
| A�� | ���ͱ��� | B�� | �����2��2-�������� | ||
| C�� | �Ҵ����Ҷ��� | D�� | 1��1-���������1��2-�������� |
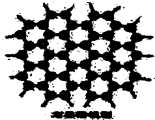 ʯī�����Dz�״�ṹ����ÿһ���ÿһ��̼ԭ�Ӷ�������3��̼ԭ�����ϣ���ͼ��ʯī�ľ���ṹ����ͼ��ͼ��ÿ���ڵ��ʾ1��̼ԭ�ӣ������ڵ������߱�ʾ1�����ۼ�����ʯī������̼ԭ�Ӹ����빲�ۼ�����֮��Ϊ��������
ʯī�����Dz�״�ṹ����ÿһ���ÿһ��̼ԭ�Ӷ�������3��̼ԭ�����ϣ���ͼ��ʯī�ľ���ṹ����ͼ��ͼ��ÿ���ڵ��ʾ1��̼ԭ�ӣ������ڵ������߱�ʾ1�����ۼ�����ʯī������̼ԭ�Ӹ����빲�ۼ�����֮��Ϊ��������| A�� | 1��3 | B�� | 2��3 | C�� | 2��1 | D�� | 3��2 |
| A�� | �縺�ԣ�X��Y | |
| B�� | ���ڱ��У�X������Y���ұ� | |
| C�� | ��X��Y�γɻ������X�����ۣ�Y�Ը��� | |
| D�� | ��̬�⻯����ȶ��ԣ�HmYǿ��HnX |
| A�� | KO2 | B�� | K2O2 | C�� | K2O3 | D�� | K2O |
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��� | �Թ�A�в��ٲ������� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ���� 5mL 5%H2O2��Һ�� 5mL10%H2O2��Һ | �Թ�A��B�о�δ�����ݲ��� |
��2��ʵ��ٵ�Ŀ�����о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죬ʵ���еμ�FeCl3��Һ��Ŀ���Ǽӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲죮
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ�����ǽ���֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/L FeCl3��Һ���۲�������ݵ����ʣ���ʵ�������ṩ�ļ����Լ�����
 ��
��