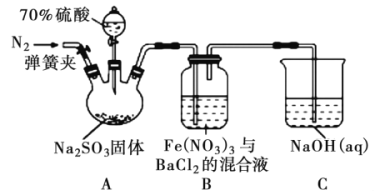
��Ŀ����
����Ŀ��������1����25 ����101 kPa�£�1 g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�������Ȼ�ѧ����ʽ��___________________________________��
��2��Zn(s) + 1/2 O2(g) = ZnO(s) ��H1 = -351 kJ/mol Hg(l) + 1/2 O2(g) = HgO(s) ��H2 = -91 kJ/mol���ɴ˿�֪ZnO(s) + Hg(l) = Zn(s) + HgO(s) ��H3= __________kJ/mol��
������������һ����Ҫ�����ȼ�ϣ����������������������Գ��������ƻ����á���ҵ�Ͽ�����ˮú���ϳɶ����ѣ��ܷ�ӦΪ��3H2(g) + 3CO(g) ![]() CH3OCH3(g) + CO2(g)����H = -246.4 kJ/mol
CH3OCH3(g) + CO2(g)����H = -246.4 kJ/mol
��1����һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⡣�ı���������֮һ��������߷�Ӧ���ʣ��������CO��ת���ʵ���________������ĸ���ţ���
a �����¶� b ������� c ��С������� d ����H2��Ũ��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK = _______________________�������¶ȣ�ƽ�ⳣ��___________�����������������С��������������
��3����һ����ɱ����ܱ������г���3 mol H2��3 mol CO��1 mol CH3OCH3��1 mol CO2����һ���¶Ⱥ�ѹǿ�·�����Ӧ��3 H2(g) + 3CO(g) ![]() CH3OCH3(g) + CO2(g)���ﵽƽ����ƽ��ʱ�����������ʵ�����ͬ��ͬѹ����ʼʱ��1.2����
CH3OCH3(g) + CO2(g)���ﵽƽ����ƽ��ʱ�����������ʵ�����ͬ��ͬѹ����ʼʱ��1.2����
�� ��Ӧ��ʼʱ�����淴Ӧ���ʵĴ�С��v(��)________v(��)��������������������������
�� ƽ��ʱ��n(CH3OCH3) = ________��ƽ��ʱCO2��ת����Ϊ________��
�� ƽ��ʱ������ԭ������Ͷ��3 mol H2��3 mol CO��1 mol CH3OCH3��1 mol CO2��һ��ʱ���ﵽ�µ�ƽ�⣬��ʱCO2��ת������ԭƽ�����________�������������� ����С���� ������������
���𰸡�CH4(g) + 2O2(g) = CO2(g) + 2H2O(l) ��H = -880 kJ/mol + 260 kJ/mol cd ![]() ��С �� 0.6 mol 40% ����
��С �� 0.6 mol 40% ����
��������
I.����ȼ���ȸ����˹������д�Ȼ�ѧ����ʽ��II.������Ի�ѧƽ���Ӱ�죬������Ӧ��ת���ʡ���ѧƽ�ⳣ���ı仯��ͬ��ͬѹ�µ�Чƽ��Ĺ��ɼ�Ӧ�á�
������1��ȼ���ȵĶ����й涨��ȼ������ʵ���Ϊ1mol����ʾȼ���ȵ��Ȼ�ѧ����ʽ�п�ȼ��Ļ�ѧ����������Ϊ1����25 ����101 kPa�£���ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4(g) + 2O2(g) = CO2(g) + 2H2O(l) ��H = -880 kJ/mol ��
��2����Zn(s) + 1/2O2(g) = ZnO(s) ��H1 = -351 kJ/mol ��Hg(l) + 1/2O2(g) = HgO(s) ��H2 = -91 kJ/mol�����������ɵ�ZnO(s) + Hg(l) = Zn(s) + HgO(s) ��H3=+ 260 kJ/mol��
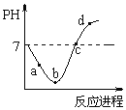
������1��������Է�Ӧ���ʡ���ѧƽ���Ӱ�죬�����¶�ʹ��Ӧ���ʼ�С����ѧƽ�����ƣ� CO��ת�������������ʹ��Ӧ����������ѧƽ�ⲻ�ƶ���CO��ת���ʲ��䣻 ��С�����������������Ũ�ȣ���Ӧ��������ѧƽ�����ƣ�CO��ת������������H2��Ũ��ʹ��Ӧ��������ѧƽ�����ƣ�CO��ת��������ѡcd��
��2���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ![]() ��������Ӧ��H<0�������¶ȣ�ƽ�����ƣ�Kֵ��С��
��������Ӧ��H<0�������¶ȣ�ƽ�����ƣ�Kֵ��С��
��3���������⣬����ʼ��ƽ���������ʵ���������Ӧ��3H2(g) + 3CO(g)![]() CH3OCH3(g) + CO2(g)��������У���ʼʱ�����淴Ӧ����v(��)��v(��)��
CH3OCH3(g) + CO2(g)��������У���ʼʱ�����淴Ӧ����v(��)��v(��)��
�������ʼ��ƽ��ʱ������������CO2���ʵ���Ϊx mol������
3H2(g) + 3CO(g)![]() CH3OCH3(g) + CO2(g)
CH3OCH3(g) + CO2(g)
��ʼ/mol�� 3 3 1 1
ת��/mol�� 3x 3x x x
ƽ��/mol�� 3+3x 3+3x 1-x 1-x
�����⣬(3+3x)+(3+3x)+(1-x)+(1-x)=1.2��(3+3+1+1)
���x��0.4
��ƽ��ʱn(CH3OCH3) =(1��x)mol=0.6mol��CO2��ת����=(x/1)��100%=40%��
��ƽ��ʱ����������Ͷ����3 mol H2��3 mol CO��1 mol CH3OCH3��1 mol CO2����������ƽ�⣬�൱����ʼʱ��6 mol H2��6 mol CO��2 mol CH3OCH3��2 mol CO2��������ƽ�⡣ͬ��ͬѹʱ��ƽ���Ч����CO2��ת���ʲ��䡣

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д�����Ŀ������ˮ��ɽ���ǽ�ɽ��ɽ��������о�NOx��SO2�ȴ�����Ⱦ������ƴ���������Ҫ���塣
(1)SO2���ŷ���Ҫ������ú��ȼ�գ���ҵ�ϳ��ð�ˮ���շ�����β���е�SO2��
��֪���չ�������ط�Ӧ���Ȼ�ѧ����ʽ���£�
��SO2(g)+NH3��H2O(aq)=NH4HSO3(aq) ��H1=a kJ/mol��
��NH3��H2O(aq)+ NH4HSO3(aq)=(NH4)2SO3(aq)+H2O(l) ��H 2=b kJ/mol��
��2(NH4)2SO3(aq)+O2(g)=2(NH4)2SO4(aq) ��H 3=c kJ/mol��
��Ӧ2SO2(g)+4NH3��H2O(aq)+O2(g)=2(NH4)2SO4(aq)+2H2O(l)����H =______kJ/mol��
(2)ȼú���糧�����÷�Ӧ2CaCO3(s)+2SO2(g)+O2(g)![]() 2CaSO4(s)+2CO2(g) ��H =-681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪT Kʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
2CaSO4(s)+2CO2(g) ��H =-681.8 kJ/mol��ú����������������SO2���ŷš����ڸ÷�Ӧ�����¶�ΪT Kʱ��������������÷�Ӧ�ڲ�ͬʱ����ϸ����ʵ�Ũ�����£�
ʱ��/min Ũ��/mol/L | 0 | 10 | 20 | 30 | 40 | 50 |
O2 | 1.00 | 0.79 | 0.60 | 0.60 | 0.64 | 0.64 |
CO2 | 0 | 0.42 | 0.80 | 0.80 | 0.88 | 0.88 |
��0~10 min�ڣ�ƽ����Ӧ����v(SO2)=___________mol/(L��min)��
��30 min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⡣�����ϱ��е������жϣ��ı������������___________������ĸ����
A������һ�����ķ�״̼��� B��ͨ��һ������O2
C���ʵ���С��������� D��������ʵĴ���
(3)NOx���ŷ���Ҫ����������β�����ɲ���NSR(NOx���滹ԭ)���д�����NOx�Ĵ���ͻ�ԭ�ڲ�ͬʱ�ν�����У���ͼa��ʾ��

��ͨ��BaO��Ba(NO3)2���ת��ʵ��NOx�Ĵ���ͻ�ԭ������NOx��������_________��
��NOx����ת��ΪBa(NO3)2�����У��μӷ�Ӧ��NO��O2�����ʵ���֮��Ϊ_________��
(4)�������÷�ӦC(s)+2NO(g)![]() N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO�������������ܱ������м���������C��һ������NO���壬���ֺ�ѹ�ڲ�ͬ�¶��·����÷�Ӧ�����ֱ���t��ʱ���NO��ת���ʣ���ͼ��ʾ��
N2(g)+CO2(g) ��H=34.0 kJ/mol���û���̿��NO�������������ܱ������м���������C��һ������NO���壬���ֺ�ѹ�ڲ�ͬ�¶��·����÷�Ӧ�����ֱ���t��ʱ���NO��ת���ʣ���ͼ��ʾ��

����ͼ��֪��1050 Kǰ��Ӧ��NO��ת�������¶����߶�������ԭ��Ϊ _______________����1100 Kʱ��CO2���������Ϊ___________��
����ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��������Kp������1050K1.1��106 Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=______________________��
[��֪�������ѹ(P��)=������ѹ(P)���������]
����Ŀ�������ü�������NO2��Ⱦ�����о���CH4+2NO2 ![]() N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ��±���
N2+CO2+2H2O����1L�ܱ������У����Ʋ�ͬ�¶ȣ��ֱ����0.50molCH4��1.2molNO2�����n(CH4)��ʱ��仯���й�ʵ�����ݼ��±���
��� | �¶� | ʱ��/min n/mol | 0 | 10 | 20 | 40 | 50 |
�� | T1 | n(CH4) | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
�� | T2 | n(CH4) | 0.50 | 0.30 | 0.18 | �� | 0.15 |
����˵����ȷ����
A. �����У�0~20min�ڣ�NO2�Ľ�������Ϊ0.0125 mol��L-1��min-1
B. ��ʵ�����ݿ�֪ʵ����Ƶ��¶�T12
C. 40minʱ��������T2Ӧ�������Ϊ0.18
D. 0��10min�ڣ�CH4�Ľ������ʢ�>��