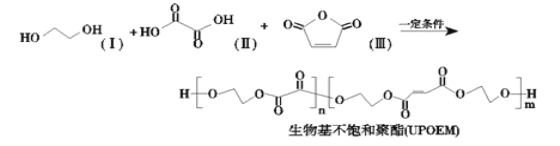
��Ŀ����
����Ŀ�����������з�Ӧ�ϳ������谷��CaO��3C![]() CaC2��CO����CaC2��N2
CaC2��CO����CaC2��N2![]() CaCN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[
CaCN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[![]() ]�����غϳ������谷��
]�����غϳ������谷��
��1��д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ��____________________________��CaCN2��������ΪCN![]() ����CN22����Ϊ�ȵ�����ķ�����CO2��________���ѧʽ�����ɴ˿�����֪CN22���Ŀռ乹��Ϊ_______��
����CN22����Ϊ�ȵ�����ķ�����CO2��________���ѧʽ�����ɴ˿�����֪CN22���Ŀռ乹��Ϊ_______��
��2�����ط�����Cԭ�Ӳ�ȡ________�ӻ���
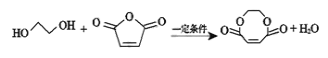
��3�������谷( )�׳������������������������谷���������� (
)�׳������������������������谷���������� (![]() )�����������������谷�����֮��ͨ��__________��ϣ������������γɽ�ʯ��
)�����������������谷�����֮��ͨ��__________��ϣ������������γɽ�ʯ��
��4��CaO������ͼ��ʾ��CaO������Ca2������λ��Ϊ________��Ca2����ȡ�Ķѻ���ʽΪ____________________________��ÿ��Ca2����Χ�����������ȵ�Ca2����________����
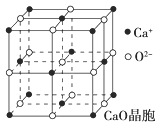
��5��CaO�����MgO����ľ����ֱܷ�Ϊ��CaO 3401 kJ��mol-1��MgO 3916 kJ��mol-1���������߾����ܲ������Ҫԭ����___________________________________��
��6����λ������K3[Fe(CN)n]���������ӻ������ɫ��������˿����ڼ����������ӣ���֪��ԭ�ӵ������������������ṩ������֮��Ϊ14����n=______________
���𰸡�1s22s22p63s23p63d104s2��[Ar]3d104s2 N2O ֱ���� sp2 (���Ӽ�)��� 6 �����������ܶѻ� 12 ���־����ж���O2-���ӣ��������ӵĴ�������ͬ�����뾶Ca2+����Mg2+����Ϊ���Ӽ���ǿ��������������ɳ����ȣ���뾶�ɷ��ȣ�����MgO�����е����Ӽ���ǿ����MgO����ľ����ܸ��ߡ� 6
��������
�Ժϳ������谷�Ļ�ѧ��ӦΪ�زģ��������ʽṹ������֪ʶ���ݹ���ԭ��д�����Ų�ʽ���õȵ���ԭ���жϿռ乹�ͣ�������ԭ�ӵļ۲���Ӷ����ж��ӻ����ͣ��ݾ����ṹȷ����λ�������Ӷѻ���ʽ����Ӱ�쾧���ܵ����ؽ��;����ܵIJ��졣
��1�����ڱ��У����ڵ������ڡ������2���ӣ�ͬ���ڡ������2���ӡ��ڲ��������ӵ�ֻ��пԪ�ص�ԭ�ӣ�������Ų�ʽΪ1s22s22p63s23p63d104s2��[Ar]3d104s2����д�ȵ����壬�ɽ�ͬ��Ԫ��ֱ���滻��������Ԫ���滻������ʧһ����Ŀ�ĵ��ӣ�����CO2��д��COS��CS2��CNO����SCN����N2O�ȡ���Ϊ�ȵ�����ķ��ӻ����ӽṹ���ƣ���CN22����CO2�ȶ���ֱ���Ρ�
��2������[![]() ]�����У�̼ԭ�ӵļ۲���Ӷ���Ϊ3���������Ӷ�3�ԡ��µ��Ӷԣ���Ϊsp2�ӻ���
]�����У�̼ԭ�ӵļ۲���Ӷ���Ϊ3���������Ӷ�3�ԡ��µ��Ӷԣ���Ϊsp2�ӻ���
��3�������谷��������������ж�����ԭ���뵪ԭ��ֱ����������ԭ�ӡ���ԭ���϶��йµ��Ӷԣ����ǿ��γɷ��Ӽ�������Ӷ��ڶ�����������γɽ�ʯ��
��4����CaO������������Ca2����������ǰ��Ⱦ����λ�ö���O2������Ca2������λ��Ϊ6��Ca2��λ�ھ�����������ģ����ѻ���ʽΪ�����������ܶѻ��������У�����O2����Χ�����O2����12������ÿ��Ca2����Χ�����������ȵ�Ca2����12����
��5��Ӱ�쾧���ܴ�С�����������ӵ���������Ӱ뾶��CaO���塢MgO���������ӵ������ͬ�������Ӱ뾶Ca2+����Mg2+��ʹ������CaO����С��MgO���塣
��6����λ������K3[Fe(CN)n]��������CN�����γ���λ��ʱÿ�������ṩһ�Ե��ӣ����ṩ2n�����ӡ�26��Ԫ���������Ų�Ϊ[Ar]3d64s2������������Ϊ2������2n+2��14��n��6��

����Ŀ���������⡿����ȩ������ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶȣ��ڼ��������·����绯��Ӧ�����Ʊ�����ȩ����ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶȣ��������ᡣ��Ӧԭ�����£�
2C6H5CHO+NaOH![]() C6H5CH2OH+C6H5COONa
C6H5CH2OH+C6H5COONa
C6H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
������������������±���
����ȩ | ���״� | ������ | �� | |
�е�/�� | 178 | 205 | 249 | 80 |
�۵�/�� | 26 | -15 | 122 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ���������£�
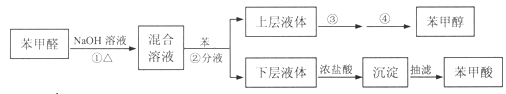
��1���ڢ�����������1Сʱ����ͼ1�������м��Ȼ�Ϲ̶�װ��Ϊ������
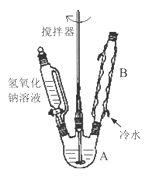
����A������Ϊ_______����������B��Ϊ����C��Ч������B��˵��ԭ��_______��
��2���������йط�Һ©����ʹ�ò���ȷ����_______
A.��Һ©����ʹ��֮ǰ��������Ƿ�©ˮ
B.��Һ©���ڵ�Һ�岻�ܹ��࣬����������
C.�����Һ©����������̨�Ͼ��ã��ֲ���������������з�Һ
D.��Һʱ���²�Һ�����������ر������������ձ��ٴ�����ʹ�ϲ�Һ������
��3�����������÷�ˮԡ���������ٽ��в����ܣ���ͼ2�����ռ�______�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ_____________��
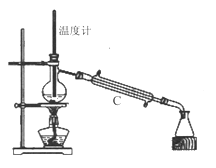
��4������ʱ����ͼ3���ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��_____��ϴ�����ϲ����ľ��塣������ɺ���������ˮ�Ծ������ϴ�ӣ�ϴ��Ӧ____________��
��5���õ�����ƽȷ��ȡ0.2440g����������ƿ�м�100mL����ˮ�ܽ⣨��Ҫʱ���Լ��ȣ�������0.1000mol/L�ı�����������Һ�ζ��������ı�����������Һ19.20mL��������Ĵ���Ϊ_____%��