��Ŀ����
19�������йؼ���ķ�����ȷ���ǣ�������| A�� | ij��Һ100mL�����к�����0.03mol������0.04mol�����ڸ���Һ��Ͷ��1.92 gͭ���ȣ���Ӧ��ų�һ����������ԼΪ0.015mol | |
| B�� | ����ʱ�����ݻ�Ϊa mL���Թ��г���NO2���壬Ȼ������ˮ�е�����ˮ�治������ʱΪֹ����ͨ��b mL O2�������Һ���ּ�������������Թ������ʣ������Ϊc mL���Ҹ����岻��֧��ȼ�գ���a��b�Ĺ�ϵ��a=4b+c | |
| C�� | ��֪ij�¶�Ksp��Ag2S��=6��10-50��Ksp��AgCl��=2��10-6����2AgCl��s��+S2-��aq��?Ag2S��s��+2Cl-��aq����ƽ�ⳣ��ԼΪ6.7��10-37 | |
| D�� | ��25���£���2a mol•L-1�İ�ˮ��0.02 mol•L-1������������ϣ���Ӧ��ȫʱ��Һ��c��NH4+��=c��Cl-�������ú�a�Ĵ���ʽ��ʾNH3•H2O�ĵ��볣��Kb=$\frac{10-9}{a-0.01}$ |
���� A��ͭ������ķ�Ӧ���ɵ������Σ�����������ʱ��NO3-��H+����ϡ���ỷ���У�ͭ���Լ�����ϡ����������ϡ���ᱻ��ԭΪNO���壮���Կ�����Cu��H+��NO3-����֮��ķ�Ӧʵ�������
B�����ݷ�Ӧ3NO2+H2O=2HNO3+NO��4NO+3O2+2H2O=4HNO3���м��㣬ע�����ʣ�����岻֧��ȼ�գ�ӦΪNO��
C������ƽ�ⳣ���Ĺ�ʽ���㣻
D����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.01mol/L�����������غ��c��NH3��H2O��=��a-0.01��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ������������NH3•H2O�ĵ��볣��Kb��
��� �⣺A��ͭ��ϡ���ᷴӦ��ʵ����3Cu+8H++2NO3-=3Cu2++2NO��+4H2O������Һ��ÿ3molCu��8molH+��ȫ��Ӧ����2molNO���壬���л����Һ�к�H+���ʵ���Ϊ��0.03mol�����к�0.06mol��0.04mol�����к�0.04mol��������Һ�й���0.10molH+��1.92gͭ�����ʵ���Ϊ0.03mol���������ӷ���ʽ���Ĺ�ϵ��3Cu2+��8H+��2NO�������ӹ�����ͭ������ȫ��Ӧ�����ɵ�NO������ͭ�����ʵ�������ó����������ʵ���Ϊ0.02mol����A����
B�����ݷ�Ӧ3NO2+H2O=2HNO3+NO��a mL NO2������$\frac{a}{3}$mL NO��ͨ��O2��������岻֧��ȼ�գ���cLΪNO������b mL O2��Ӧ��NOΪ��$\frac{a}{3}$-c��mL�����ݷ���ʽ4NO+3O2+2H2O=4HNO3����$\frac{a}{3}$-c����b=4��3�������ɵ�a=4b+3c����B����
C��2AgCl��s��+S2-��aq��?Ag2 S��s��+2C1- ��aq����ƽ�ⳣ��K=$\frac{c��C{l}^{-}��^{2}}{c��{S}^{2-}��}$=$\frac{\frac{{K}^{2}sp��AgC1��}{{c}^{2}��A{g}^{+}��}}{\frac{Ksp��A{g}_{2}S��}{{c}^{2}��A{g}^{+}��}}$=$\frac{��2��1{0}^{-6}��^{2}}{6��1{0}^{-50}}$=6.7��1037����C����
D����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.01mol/L�����������غ��c��NH3��H2O��=��a-0.01��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ�NH3•H2O�ĵ��볣��Kb=$\frac{c��O{H}^{-}��•c��N{H}_{4}^{+}��}{c��N{H}_{3}•{H}_{2}O��}$=$\frac{1{0}^{-7}��1{0}^{-2}}{a-0.01}$=$\frac{1{0}^{-9}}{a-0.01}$����D��ȷ��
��ѡC��
���� ���⿼��������ԭ��Ӧ�ļ��㣬Ϊ��Ƶ���㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע��������ʵ����ʣ�Ϊ������Ĺؼ����ѶȲ���

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
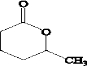
Сѧͬ�����������ܾ�ϵ�д�| A�� | ��A��NaHCO3��Һ���������ɣ���A�Ľṹ����4�� | |
| B�� | ��A��ϡ���������������л����A�Ľṹ����4�� | |
| C�� | ��A�ܷ���������Ӧ����A�Ľṹ��2�� | |
| D�� | ��A���ܺ�����Cu��OH��2��Ӧ����ש��ɫ���������ܺͽ����Ʒ�Ӧ�ų�H2����A�Ľṹ��3�� |
| A�� | Na2CO3��Һ��ˮϡ�ͺָ���ԭ�¶ȣ�pH��Kw����С | |
| B�� | NH4Cl��CH3COONa��NaHCO3��NaHSO3����ˮ����ˮ�ĵ��붼�дٽ����� | |
| C�� | Ũ�Ⱦ�Ϊ0.10mol•L-1 NH4Cl��NH4HSO4��Һ��c��NH4+��ǰ�ߴ��ں��� | |
| D�� | ����Na2CO3��Һ����ˮ���е�CaSO4��ת��Ϊ���ڳ�ȥ��CaCO3 |
| A�� | ���Ʒ�Ӧ�ų����� | B�� | �������ᷢ��������Ӧ | ||
| C�� | �ܷ���������Ӧ | D�� | ����С�մ���Һ��Ӧ���������� |
| A�� | ����CH3COONa����Һ��������ˮ�ĵ���̶� | |
| B�� | pH=5��HCl��pH=5��NH4Cl�������ϣ���ҺpH��Ϊ5 | |
| C�� | ��AgI��AgBr�ı�����Һ�������ϣ��ټ�������ŨAgNO3��Һ�������ij�����Ҫ��AgBr | |
| D�� | 20mL0.1mol/LNH3•H2O��10mL0.1mol/LHCl��ַ�Ӧ��������ҺpH=9��c��NH3•H2O����c��Cl-����c��OH-�� |
| A�� | ʣ����������ͭ����� | |
| B�� | ��Ӧ����Һ��n��Fe3+��=0.10mol | |
| C�� | ԭ����������ͭ��������9.6g | |
| D�� | ��Ӧ����Һ��n��Fe2+��+n��Cu2+��=0.64mol |
| A�� | ������������ʳƷ������ | |
| B�� | �ѻ�������ֲ����ʹ��ˮ��ɫ��ԭ����ͬ | |
| C�� | �Ͼɵ���к����ؽ������ӣ���Ӧ���л��գ������� | |
| D�� | �������pH��7����ˮ����Ҫ���ɴ����е�SO2��NO2����ɵ� |
| A�� | Ԫ��Y��Z��W�γɵļ����Ӿ�����ͬ���Ӳ�ṹ�������Ӱ뾶�������� | |
| B�� | X��Z��R����Ԫ�ع��ɵĻ�������ֻ�����Ӽ� | |
| C�� | Ԫ��Z��R�������Ӧ��ˮ����֮�����Ӧ�����ɵ�����Һֻ�ܳ����� | |
| D�� | Ԫ��Y��̬�⻯����ȶ���ǿ��Ԫ��R |
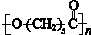 ����ϳ�·�����£�
����ϳ�·�����£�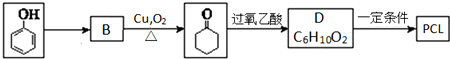

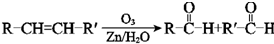
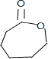 ����������D�ķ�Ӧ������������Ӧ��
����������D�ķ�Ӧ������������Ӧ��
 ��
�� ��XOHCCH2CH2CH2CH2CHO��
��XOHCCH2CH2CH2CH2CHO��