��Ŀ����
14����A��B��C��D��E����Ԫ�أ�����A��B��C����ͬһ���ڣ�Aԭ�������p�ܼ��ĵ��������ڴ����ĵ���������BԪ�ؿɷֱ���A��C��D��E����RB2�ͻ������֪��DB2��EB2�У�D��B��������Ϊ7��8��E��B��������Ϊ1��1�����������������ش��������⣺��1������C��ԭ�ӽṹʾ��ͼ
 ��
����2��д��Dԭ�ӵļ۵����Ų�ʽ3s23p2��
��3��д��AԪ����B����ȫȼ�յĻ�ѧ����ʽC+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2��
��4��ָ��EԪ����Ԫ�����ڱ��е�λ�õ������ڢ�A�壮
��5���Ƚ�A��B��C����Ԫ�صĵ�һ�����ܵĴ�С˳��N��O��C�����ɴ�С��˳�����У���Ԫ�ط��ű�ʾ����
��6���Ƚ�Ԫ��D��E�ĵ縺�Ե���Դ�СSi��S��
���� ��A��B��C��D��E����Ԫ�أ�Aԭ�������p�ܼ��ĵ��������ڴ����ĵ���������p�ܼ�������������6����ԭ��ֻ����2�����Ӳ㣬��������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�
A��B��C����ͬһ���ڣ�BԪ�ؿɷֱ���A��C��D��E����RB2�ͻ����BӦΪOԪ�أ�CΪNԪ�أ���DB2��EB2�У�D��B��������Ϊ7��8������Mr��D����Mr��O��=7��4����M��D��=7��$\frac{16}{4}$=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����Mr��E��=2M��O��=2��16=32������EΪSԪ�أ��ݴ˽��
��� �⣺��A��B��C��D��E����Ԫ�أ�Aԭ�������p�ܼ��ĵ��������ڴ����ĵ���������p�ܼ�������������6����ԭ��ֻ����2�����Ӳ㣬��������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�A��B��C����ͬһ���ڣ�BԪ�ؿɷֱ���A��C��D��E����RB2�ͻ����BӦΪOԪ�أ�CΪNԪ�أ���DB2��EB2�У�D��B��������Ϊ7��8������Mr��D����Mr��O��=7��4����M��D��=7��$\frac{16}{4}$=28��DӦΪSiԪ�أ�E��B��������Ϊ1��1����Mr��E��=2M��O��=2��16=32������EΪSԪ�أ�
��1��CΪNԪ�أ���ԭ�ӽṹʾ��ͼ�� ��
��
�ʴ�Ϊ�� ��
��
��2��DΪSiԪ�أ�ԭ������Ϊ14����ԭ�ӵļ۵����Ų�ʽ3s23p2��
�ʴ�Ϊ��3s23p2��
��3��̼����������ȫȼ�����ɶ�����̼����Ӧ����ʽΪ��C+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2��
�ʴ�Ϊ��C+O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2��
��4��EΪSԪ�أ��������ڱ��е������ڢ�A�壬
�ʴ�Ϊ���������ڢ�A�壻
��5��ͬ������ԭ����������һ�����ܳ�����ǿ�ᣬ����Nԭ��2p���Ϊ�����״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ����Ե�һ������N��O��C��
�ʴ�Ϊ��N��O��C��
��6��Si��Sͬ���ڣ���ԭ���������縺�����ʵ縺��Si��S��
�ʴ�Ϊ��Si��S��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ���ע����������ͬ����Ԫ�ص�һ�������쳣�����

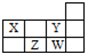
| A�� | 1��37Wԭ���У�����������������3�� | |
| B�� | Y��W����ͬһ���壬������ͬ�����̬ | |
| C�� | Z�γɼ������Ӱ뾶С��W�γɼ������Ӱ뾶 | |
| D�� | X��Y��Z��Wÿ������Ԫ�ؼ�����γɵ�������ȵ��⻯�� |
| A�� | ԭ�Ӱ뾶��r��Z����r��M����r��N����r��Y����r��X�� | B�� | Ԫ�صĸ����ԣ�Y��N��X��Z | ||
| C�� | Ԫ�صĵ�һ�����ܣ�I1��M����I1��Z����I1��X�� | D�� | ԭ���е�Ϊ�ɶԵ�������N��Y��Z��M |

��Щģ�͵���ʷ�ݱ�˳���ǣ�������
| A�� | �٢ڢۢܢ� | B�� | �٢ۢڢݢ� | C�� | �٢ݢۢڢ� | D�� | �٢ۢݢܢ� |
| A�� | �������м�����������Һ��Cl-+Ag+�TAgCl�� | |
| B�� | ����������������ˮ��Һ���ȣ�CH3CH2Br+NaOH$��_{��}^{H_{2}O}$CH2�TCH2��+NaBr+H2O | |
| C�� | ��ϩ�ۺϣ�nCH2�TCHCH3$��_{��}^{����}$ | |
| D�� | ��������Һ��ͨ������������̼��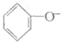 +CO2+H2O�� +CO2+H2O�� +HCO3- +HCO3- |

| A�� | ������Һ�����Ϊ30 mL | |
| B�� | bʱ����Һ��SO42-��Ũ��ԼΪ0.125 mol•L-1 | |
| C�� | dʱ����Һ��pHԼΪ13 | |
| D�� | ��Һ�ĵ���������c��d=b��a |
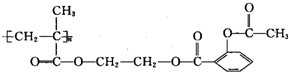 ��1mol����������NaOH��Ӧ�����ʵ����ǣ�������
��1mol����������NaOH��Ӧ�����ʵ����ǣ�������| A�� | 3mol | B�� | 4mol | C�� | 3n mol | D�� | 4n mol |
| A�� | �ȷֽⷨ | B�� | �Ȼ�ԭ�� | C�� | ���ȼ��� | D�� | ��ⷨ |
| A�� | �ŵ�ʱ�����ĵ缫��ӦΪ��CH3OH-6e-+8OH-�TCO${\;}_{3}^{2-}$+6H2O | |
| B�� | ���ʱ�������Һ��pH������ | |
| C�� | �ŵ�ʱCH3OH���뷴Ӧ�ĵ缫Ϊ���� | |
| D�� | ���ʱÿ����1 mol CH3OHת��6 mol���� |