��Ŀ����
4���ݱ��������Ħ��������˾�з���һ���ɼ״��������Լ�ǿ�����������Һ�������ֻ���أ������ɴ�����ʹ�õ������ػ�﮵�ص�ʮ�������ط�ӦΪ��2CH3OH+3O2+4OH-$?_{���}^{�ŵ�}$2CO32-+6H2O��������˵��������ǣ�������| A�� | �ŵ�ʱ�����ĵ缫��ӦΪ��CH3OH-6e-+8OH-�TCO${\;}_{3}^{2-}$+6H2O | |
| B�� | ���ʱ�������Һ��pH������ | |
| C�� | �ŵ�ʱCH3OH���뷴Ӧ�ĵ缫Ϊ���� | |
| D�� | ���ʱÿ����1 mol CH3OHת��6 mol���� |
���� �ŵ�ʱ����ԭ�����ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫��������������ӦʽΪCH3OH-6e-+8OH-�TCO32-+6H2O��������ӦʽΪO2+4e-+2H2O=4OH-�����ʱ�������������缫��Ӧʽ��������������Ӧʽ�����෴���ݴ˷������
��� �⣺A���ŵ�ʱ����ԭ�����ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫��������������ӦʽΪCH3OH-6e-+8OH-�TCO32-+6H2O��������ӦʽΪO2+4e-+2H2O=4OH-����A��ȷ��
B�����ݵ�ط�Ӧʽ֪�����ʱ�����������������ɣ�������Һ������ǿ����B��ȷ��
C��ȼ�ϵ���У�ͨ��ȼ�ϵĵ缫�Ǹ�����ͨ���������ĵ缫������������ͨ��״��ĵ缫�Ǹ�������C����
D�����ʱÿ����1molCH3OHת��1mol����4+2��=6mol���ӣ���D��ȷ��
��ѡC��
���� ���⿼�黯ѧ��Դ���͵�أ�Ϊ��Ƶ���㣬��ȷ�����缫�Ϸ����ķ�Ӧ�ǽⱾ��ؼ����ѵ��ǵ缫��Ӧʽ����д����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
15����Ӱ��ˮ�ĵ���ƽ�⣬��ʹ��Һ��c��H+����c��OH-���IJ����ǣ�������
| A�� | ��ˮ��Ͷ��һС������� | B�� | ��ˮ������� | ||
| C�� | ��ˮ�м���CH3COONa���� | D�� | ��ˮ�м���NH4Cl��Һ |
12�����ڿ��淴Ӧ�ﵽƽ��״̬��˵��������ǣ�������
| A�� | ��ƽ��ʱͬһ���ʵ�V������=V���棩 | B�� | �Ƕ�̬ƽ�� | ||
| C�� | ����ֵĺ�������ʱ����仯 | D�� | ����������ܸı�ƽ�� |
19�����л�ѧ������д��ȷ���ǣ�������
| A�� | ��ԭ�ӵĽṹʾ��ͼ��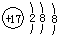 | B�� | CH4���ӵ����ģ�ͣ�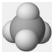 | ||
| C�� | �Ȼ�þ�ĵ���ʽ��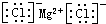 | D�� | N2�Ľṹʽ��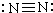 |
9������˵���У���ȷ���ǣ�������
| A�� | 1 mol H2SO4��1 molBa��OH��2��Ӧ����H2Oʱ�ų����Ƚ����к��� | |
| B�� | ��H��0 kJ•mol-1��ʾ���ȷ�Ӧ����H��0 kJ•mol-1��ʾ���ȷ�Ӧ | |
| C�� | �Ȼ�ѧ����ʽ�еĻ�ѧ��������ʾ���ʵ����������Ƿ��� | |
| D�� | 1 mol H2��0.5 mol O2��Ӧ�ų����Ⱦ���H2��ȼ���� |
16���±���Ԫ�����ڱ��ж�����Ԫ�ز��֣�������ĸ�ֱ����һ��Ԫ�أ�
��1��������ѧ��������õ�Ԫ���Ǻ�����Ԫ�����ƣ���
��2������h��ԭ�ӽṹʾ��ͼ ���䵥���ڵ��ӹ�ҵ������Ҫ��;��д����ҵ��ȡ�õ��ʵĻ�ѧ����ʽ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO����
���䵥���ڵ��ӹ�ҵ������Ҫ��;��д����ҵ��ȡ�õ��ʵĻ�ѧ����ʽ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO����
��3��c��d��g���Ӱ뾶��С�����˳����O2-��Na+��Al3+ �������ӷ��ű�ʾ����
��4������Ԫ��g���⻯���ȶ��ԣ��������������Ԫ��e���⻯���ȶ��ԣ�
��5��cԪ������������ˮ�����к������Ӽ������ۼ����ѧ�����ͣ��������ʲ��ܴ����ڴ����������Լ�ƿ�е�ԭ��SiO2+2NaOH=Na2SiO3+H2O���û�ѧ����ʽ��ʾ����
| a | b | ||||||
| e | f | g | |||||
| c | d | h | i | j | |||
��2������h��ԭ�ӽṹʾ��ͼ
 ���䵥���ڵ��ӹ�ҵ������Ҫ��;��д����ҵ��ȡ�õ��ʵĻ�ѧ����ʽ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO����
���䵥���ڵ��ӹ�ҵ������Ҫ��;��д����ҵ��ȡ�õ��ʵĻ�ѧ����ʽ2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO������3��c��d��g���Ӱ뾶��С�����˳����O2-��Na+��Al3+ �������ӷ��ű�ʾ����
��4������Ԫ��g���⻯���ȶ��ԣ��������������Ԫ��e���⻯���ȶ��ԣ�
��5��cԪ������������ˮ�����к������Ӽ������ۼ����ѧ�����ͣ��������ʲ��ܴ����ڴ����������Լ�ƿ�е�ԭ��SiO2+2NaOH=Na2SiO3+H2O���û�ѧ����ʽ��ʾ����
13�����и������ʿ���ֱ��ͨ����Һ���з��������ǣ�������
�ٱ���ˮ��
�������������Ҵ���
�����������ʹ�����Һ��
�ܱ����屽��
����������ˮ��
�������������ǣ�
�ٱ���ˮ��
�������������Ҵ���
�����������ʹ�����Һ��
�ܱ����屽��
����������ˮ��
�������������ǣ�
| A�� | �٢ۢ� | B�� | �٢ܢݢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
14����5.6 L����״������ϩ�ͼ���Ļ������ͨ��������ˮ�У���ַ�Ӧ����ˮ������������2.8 g��ԭ�����������ϩ�������������ǣ�������
| A�� | 2��1 | B�� | 2��3 | C�� | 7��6 | D�� | 7��3 |
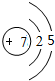 ��
��