��Ŀ����
13��SO2��NO2�������˿���������������Ҫ��ɣ���1��SO2�����������������գ�����0.4molSO2������200mL��3mol•L-1NaOH��Һ������ȫ���գ�������ΪNaHSO3��Na2SO3���ѧʽ�������ⶨ������Һ�����ԣ�����Һ������Ũ���ɴ�С��˳��Ϊ��c��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
��2��CO������ȼ�ϵ�أ���KOH��Һ������ʣ��������ֱ����CO�Ϳ��������������У�������Ӧ����ʽΪ��CO+4OH--2e-=CO32-+2H2O��
��3�����������̼�������ڴ��������¿ɷ�����Ӧ��2CO+2NO$\stackrel{����}{?}$N2+2CO2�������Ϊ0.5L���ܱ��ݻ��У�����0.40mol��CO��0.40mol��NO����Ӧ��N2�����ʵ���Ũ�ȵı仯�����ͼ1��ʾ���ӷ�Ӧ��ʼ��ƽ��ʱ��CO��ƽ����Ӧ���ʦԣ�CO��=0.05mol•L-1•min-1��

��4����CO2�ϳɶ����ѵĻ�ѧ��Ӧ�ǣ�
2CO2��g��+6H2��g���TCH3OCH3��g��+3H2O��g����H��0��
�ϳɶ�����ʱ���������������̼�����ʵ���֮��Ϊ4��1��CO2��ת������ʱ��ı仯��ϵ��ͼ2��ʾ��
��A����淴Ӧ���ʦ��棨CO2����B�������Ӧ����Ϊ������CO2�������������������=������
��������ƽ��ת����Ϊ45%��
��5��Һ����Ϊһ��DZ�ڵ��������ȼ�ϣ����ڰ�ȫ�ԡ��۸�ȷ���ϻ�ʯȼ�Ϻ���ȼ�����Žϴ�����ƣ�����ȼ��ʵ������صķ�Ӧ�У�
4NH3��g��+3O2��g��=2N2��g��+6H2O��1����H1 ��
4NH3��g��+5O2��g��=4NO��g��+6H2O��1����H2 ��
4NH3��g��+6NO��g��=5N2��g��+6H2O��1����H3 ��
��д������������Ӧ�С�H1����H2����H3����֮���ϵ�ı���ʽ����H1=$\frac{{3��{H_2}+2��{H_3}}}{5}$��
���� ��1��NaOH�����ʵ���Ϊ0.200L��3mol•L-1=0.6mol��n��SO2��=0.4mol������2NaOH+SO2�TNa2SO3+H2O��֪��������������������ֵĶ��������ٷ�����ӦNa2SO3+H2O+SO2�T2NaHSO3�����ݷ���ʽ���м�����Һ��������ɣ�
��2������������CO�ڸ����ŵ�����CO32-��
��3��ͼ���֪��Ӧ����ƽ��״̬�����ɵ���Ũ��Ϊ0.10mol/L������V=$\frac{��c}{��t}$���㵪����Ӧ���ʣ���Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȼ���õ�һ����̼��ʾ�ķ�Ӧ���ʣ�
��4������CO2��ת������ʱ��仯ͼ��֪��A��ʱ��Ӧ��δ�ﵽƽ��״̬����Ӧ�Խ�����������У�
����CO2�ij�ʼ���ʵ���Ϊa����H2�ij�ʼ���ʵ���Ϊ4a����ͼ��֪��CO2��ת����Ϊ60%����ת���Ķ�����̼Ϊ0.6a�����ݷ���ʽ����ת�������������ʵ������ٸ���ת���ʶ�����㣻
��5�������Ȼ�ѧ����ʽ��˹���ɼ���õ������Ȼ�ѧ����ʽ�����ã�3����+2���ۣ���$\frac{1}{5}$�õ���
��� �⣺��1���跴Ӧ�����������Ƶ����ʵ���Ϊx�����Ķ�����������ʵ���Ϊy��
2NaOH+SO2�TNa2SO3+H2O
2 1 1
0.6mol y x
��ã�x=0.3mol y=0.3mol
���������������֪�������������ǹ����ģ�ʣ��Ķ�����������ʵ���Ϊ��0.4mol-0.3mol=0.1mol
���Զ������������ɵ��������Ƽ�����Ӧ��
�������������Ƶ����ʵ���Ϊa���������������Ƶ����ʵ���Ϊb
Na2SO3+H2O+SO2�T2NaHSO3
1 1 2
a 0.1mol b
a=0.1mol��b=0.2mol
��Һ�к���NaHSO3���ʵ���Ϊ0.2mol��Na2SO3�����ʵ���Ϊ0.2mol
���ⶨ������Һ�����ԣ�˵�� ������������ӵ�����������������ˮ�⣬��Һ������Ũ�ȴ�СΪ��c��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
�ʴ�Ϊ��NaHSO3��Na2SO3��c��Na+����c��SO32-����c��HSO3-����c��H+����c��OH-����
��2��ԭ����У�������COʧ���Ӻ����������ӷ�Ӧ����CO32-�����Ե缫��ӦʽΪ��CO+4OH--2e-=CO32-+2H2O��
�ʴ�Ϊ��CO+4OH--2e-=CO32-+2H2O��
��3��ͼ���֪��Ӧ����ƽ��״̬�����ɵ���Ũ��Ϊ0.10mol/L������V=$\frac{��c}{��t}$���㵪����Ӧ����=$\frac{0.10mol/L}{4min}$=0.025mol/��L•min������Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȼ���õ�һ����̼��ʾ�ķ�Ӧ����V��CO��=2V��N2��=2��0.025mol/��L•min��=0.05mol•L-1•min-1��
�ʴ�Ϊ��0.05mol•L-1•min-1��
��4��2CO2��g��+6H2��g���TCH3OCH3��g��+3H2O��g����H��0��
����CO2��ת������ʱ��仯ͼ��֪��A��ʱ��Ӧ��δ�ﵽƽ��״̬����Ӧ�Խ�����������У���v����CO2��С��B��ƽ��ʱ�Ļ�ѧ��Ӧ���ʣ��ʴ�Ϊ������
����CO2�ij�ʼ���ʵ���Ϊa����H2�ij�ʼ���ʵ���Ϊ4a����ͼ��֪��CO2��ת����Ϊ60%����ת���Ķ�����̼Ϊa��60%=0.6a�����ݷ���ʽ��֪��ת����H2�����ʵ���Ϊ0.6a��$\frac{6}{2}$=1.8a����ƽ��ʱ������ת���ʶ�Ϊ$\frac{1.8a}{4a}$��100%=45%��
�ʴ�Ϊ��45%��
��5��4NH3��g��+3O2��g��=2N2��g��+6H2O��1����H1 ��
4NH3��g��+5O2��g��=4NO��g��+6H2O��1����H2 ��
4NH3��g��+6NO��g��=5N2��g��+6H2O��1����H3 ��
���ݸ�˹���ɼ��㣨3����+2���ۣ���$\frac{1}{5}$��4NH3��g��+3O2��g��=2N2��g��+6H2O��1����H1=$\frac{{3��{H_2}+2��{H_3}}}{5}$��
�ʴ�Ϊ��$\frac{{3��{H_2}+2��{H_3}}}{5}$��
���� ���⿼���˻�ѧ��Ӧ����ļ�������жϣ�ԭ��ص缫��Ӧ��д��������ѧƽ���Ӱ�����ء���˹���ɵļ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �߽�������ϵ�д�
�߽�������ϵ�д�| A�� | ��B�� | B�� | ��B�� | C�� | ��A�� | D�� | ��A�� |
| A�� | ��֪����ƽ�ⳣ����H2CO3��HClO��HCO3-����NaClO��Һ��ͨ������CO2��2ClO-+CO2+H2O�T2HClO+CO32- | |
| B�� | ��Fe��NO3��2��NaBr�����Һ�еμ�ϡ���6Br-+8H++2NO3-�T3Br2+2NO��+4H2O | |
| C�� | Na2S��Һ�еμ�NClO��Һ��S2-+ClO-+H2O�TS��+Cl-+2OH- | |
| D�� | ˫��ˮʹ����KMnO4��Һ��ɫ��2MnO4-+5H2O2�T2Mn2++5O2��+6OH-+2H2O |
| A�� | ��������ʴ�ı�����M+nH2O�TM��OH��n+$\frac{n}{2}$H2�� | |
| B�� | ��ijЩ���ߵġ���еת����λ��ѡ��ˢ����ķ��������� | |
| C�� | ������һ������·����ĵ绯ѧ��ʴ��Ҫ��������ʴ | |
| D�� | �����£����ڿ����еĽ�����Ҫ������ѧ��ʴ |
| A�� | ��ǰ����ˮϴһϴ | B�� | �����з���ľ̿����ζ��ʧ | ||
| C�� | ���г���̥�������±��� | D�� | ʳ����� |
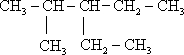 ��
��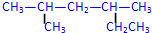 2��4-��������
2��4-��������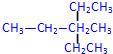 3-��-3-�һ�����
3-��-3-�һ����� +2HNO3$��_{100-110��}^{Ũ����}$
+2HNO3$��_{100-110��}^{Ũ����}$ +2H2O��
+2H2O��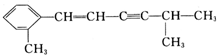 �÷�����������8��Cԭ����ͬһƽ�棬�����13��Cԭ����ͬһƽ�森
�÷�����������8��Cԭ����ͬһƽ�棬�����13��Cԭ����ͬһƽ�森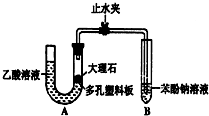 ijУѧ��С��Ϊ̽�����ᡢ̼��ͱ��ӵ�����ǿ����֤������Ϊ���ᣬ��������ʵ�飮
ijУѧ��С��Ϊ̽�����ᡢ̼��ͱ��ӵ�����ǿ����֤������Ϊ���ᣬ��������ʵ�飮
 $��_{��}^{һ������}$Y$��_{��}^{һ������}$��
$��_{��}^{һ������}$Y$��_{��}^{һ������}$��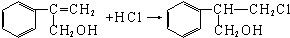 ������ע����Ӧ��������
������ע����Ӧ��������
 ��
�� ��
�� ��
��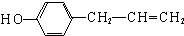 ������һ�֣���
������һ�֣��� H��C��N��Cr����ѧ��ѧ�����ļ���Ԫ�أ����ǵĵ��ʼ���������������������еȷ���Ӧ�ù㷺����ش��������⣺
H��C��N��Cr����ѧ��ѧ�����ļ���Ԫ�أ����ǵĵ��ʼ���������������������еȷ���Ӧ�ù㷺����ش��������⣺