��Ŀ����
5���������ӷ���ʽ��д��ȷ���ǣ�������| A�� | ��֪����ƽ�ⳣ����H2CO3��HClO��HCO3-����NaClO��Һ��ͨ������CO2��2ClO-+CO2+H2O�T2HClO+CO32- | |
| B�� | ��Fe��NO3��2��NaBr�����Һ�еμ�ϡ���6Br-+8H++2NO3-�T3Br2+2NO��+4H2O | |
| C�� | Na2S��Һ�еμ�NClO��Һ��S2-+ClO-+H2O�TS��+Cl-+2OH- | |
| D�� | ˫��ˮʹ����KMnO4��Һ��ɫ��2MnO4-+5H2O2�T2Mn2++5O2��+6OH-+2H2O |
���� A���ɵ���ƽ�ⳣ����H2CO3��HClO��HCO3-����NaClO��Һ��ͨ������CO2����Ӧ���ɴ�������̼�����ƣ�
B�����������ӻ�ԭ��ǿ�������ӣ���������������Ի��������������������ӣ�
C��Na2S��Һ�еμ�NClO��Һ�����߷���������ԭ��Ӧ�������Ȼ��ƺ��������ƣ�
D��������Һ�в����������������ӣ�
��� �⣺A����֪����ƽ�ⳣ����H2CO3��HClO��HCO3-����NaClO��Һ��ͨ������CO2���ӷ���ʽ��ClO-+CO2+H2O=HClO+HCO3-����A����
B���������ӵĻ�ԭ�Դ��������ӣ���Fe��NO3��2��NaBr�����Һ�еμ�ϡ�������ӷ���ʽΪFe2++Br-+4H++NO3-�TBr2+NO��+2H2O+Fe3+����B����
C��Na2S��Һ�еμ�NClO��Һ�����߷���������ԭ��Ӧ�������Ȼ��ƺ��������ƣ����ӷ���ʽΪS2-+ClO-+H2O�TS��+Cl-+2OH-����C��ȷ��
D��˫��ˮ������KMnO4��Һ��Ӧ���������ӡ�������ˮ�����ӷ���ʽΪ6H++2MnO4-+5H2O2�T2Mn2++5O2��+8H2O����D����
��ѡ��C��
���� ���⿼�������ӷ���ʽ����д����ȷ��Ӧʵ���ǽ���ؼ���ע�����ƽ�ⳣ������������ǿ���Ĺ�ϵ��ѡ��A��BΪ�״�ѡ�

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д�| A�� | ���������֧��������ӦΪ������ԭ��Ӧ���ҵ���ת������ͬ | |
| B�� | ͨ��������������ζ���жϷ�Ӧ���� | |
| C�� | ��ϡ����ɼ�����������ơ�����������Һ | |
| D�� | ���ɵ������Ư�����Ը��������Һ |
| A�� | �÷�Ӧ����������Cl2 | |
| B�� | ���ݸ÷�Ӧ���жϻ�ԭ�ԣ�S2O32-��Cl- | |
| C�� | ������Ӧ�У�ÿ����l mol SO42-��ת��4 mol���� | |
| D�� | SO2��������Ư��ԭ����ͬ������Ҳ������SO2����֯��ҵ��Ư�� |
| A�� | �������仯�Ĺ���һ���ǻ�ѧ�仯���� | |
| B�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧ�����ȷ�Ӧ | |
| C�� | ��ѧ��Ӧ��һ�����������ı仯 | |
| D�� | ���ʷ���ȼ�յķ�Ӧһ���Ƿ��ȷ�Ӧ |
| A�� | 16 g | B�� | 32 g | C�� | 16 | D�� | 32 |

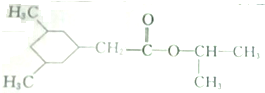 ������·���£�
������·���£�
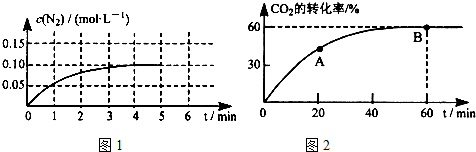
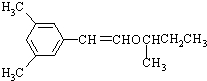 ��
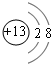
��
 ��
��