��Ŀ����
��13�֣���ͨ��״���£�X��Y��Z��������̬���ʡ�X�����Ԫ���ǵ�������ԭ�Ӱ뾶��С��Ԫ��(ϡ������Ԫ�س���)��Y��Z����Ԫ��R��ɣ���ӦY+2I-+2H+====I2+Z+H2O����ΪY�ļ�����Ӧ��W�Ƕ�����Ԫ�أ����������������ڲ���������������������ӶԵ�������X���ʵ����Ԫ��Ҫ����
��1�� Z�Ļ�ѧʽ__________________
��2����Y�Ͷ�������ֱ�ͨ��Ʒ����Һ������ʹƷ����ɫ����������ɫ����Һ����Y�Ͷ��������ʵ�鷽��:________________________________________________________��
��3���ٳ�ʵ��˵��X�������Ա�W����������ǿ(����һ����ѧ����ʽ��ʾ):_____________��
����ͼ��0.1 mol��L��1���ֵ������Һ��pH���¶ȱ仯��ͼ��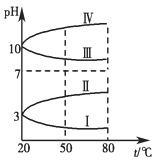
��1�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(��д���)��
��2��20 ��ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+)��3c(Al3��)��________�������㾫ȷֵ��
��3������ʱ����100 mL 0.1 mol��L��1 NH4HSO4��Һ�еμ�0.1 mol��L��1 NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��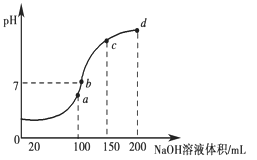
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������________�㣻��b�㣬��Һ�и�����Ũ���ɴ�С������˳����______________________
��1��O2��2�֣�
��2��������ɫ�����Һ������Һ�ָ���ɫ����ԭͨ������ΪSO2������Һ����죬��ԭͨ��������O3��2�֣�
��3�� H2S+Cl2====S��2HCl��1�֣�(���������𰸾���)
��1����2�֣�
��2����10��3-10��11��mol��L��1 ��2�֣� ��3)a��2�֣���c(Na��)��c(SO42-)��c(NH4+)��c(OH��)��c(H��) ��2�֣�
�������������I�����ȸ�����Ϣȷ��XΪCl2��RΪ��Ԫ�أ�YΪO3��ZΪO2��WΪ��Ԫ�ء�O3�����ǿ������ʹƷ����ɫ����SO2��ͬ���ʶ���ɫ�����Һ���м��ȣ�����Һ�ָ���ɫ����ԭͨ������ΪSO2������Һ����죬��ԭͨ��������O3����˵��Cl2�������Ա�S��������ǿ�ķ�Ӧ��H2S+Cl2====S��2HCl��K2S+Cl2====S��2KCl�ȡ�
���NH4Al(SO4)2��NH4+��Al3������ˮ��ʹ��Һ�����ԣ�pH��7�������¶����ߣ��ٽ�ˮ�⣬������ǿ��pH��С�����ߢ���ȷ��
�ƾ�NH4Al(SO4)2��Һ�еĵ���غ�ʽΪ2c(SO42-)+ c(OH��)��c(H+)+c(NH4+)+3c(Al3��)����2c(SO42-)��c(NH4+)��3c(Al3��)��c(H+) ��c(OH��)����1��10��3��1��10��11��mol��L��1��
����a��������ǡ�÷�Ӧ����(NH4)2SO4��NH4+����ˮ�⣬ˮ�ĵ���̶������b��[��a�㣨ǡ������(NH4)2SO4��֮�����Զ��һ��NaOH��ʹ����NH4+��OH����Ӧ����NH3��H2O����c(Na��)��c(SO42-)��c(NH4+)]���ָ�����ҺΪ���ԣ�����c(Na��)��c(SO42-)��c(NH4+)��c(OH��)��c(H��)��
���㣺���⿼��Ԫ�������ɡ��ε�ˮ�⡢����غ㡢����Ũ�ȴ�С�Ƚϵ�֪ʶ��

��7�֣������������ʵ���Ҫ���������ʽṹ����ش��������⣺
��1��ͭ�ǹ���Ԫ�ء��������У�ͭ������+1�ۻ�+2�ۡ���ͼΪijͭ�����ᄃ��ṹ��Ԫ����������Ļ�ѧʽΪ ��
��2���������ڲ���Ԫ�ط�������۵���±���
| ������ | NaF | MgF2 | SiF4 |
| �۵�/K | 1266 | 1534 | 183 |
��3��A��BΪ��������Ԫ�أ���ԭ�ӵIJ��ֵ��������±���ʾ��
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
��4���о����ʴ��Ա��������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2��Fe3O4�У����ʺ���¼�����ŷ�ԭ�ϵ���__________���ѧʽ����
��5��ʵ���Ҽ���Ni2+���ö���ͪ���֮���������Ⱥ�ɫ����������
������������û�ѧ����������δ��������������������λ��Ϊ4����
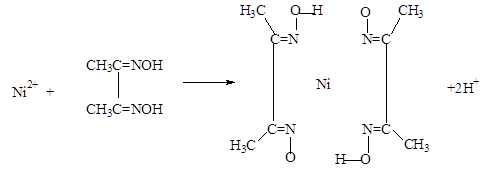
��15�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
| A | A������������Ӧ��ˮ���ﻯѧʽΪH2AO3 |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | Cԭ����ͬ����ԭ���а뾶���ϡ��������⣩���䵥����ɫΪ��ɫ |
| D | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p2 |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��Ϊ��������������������A������Ȼ��ﹹ�ɾ��������������Ϊ������������������������������
��3��A��B������⻯���н��ȶ����������������ѧʽ����B������⻯���E�ĺ�ɫ����������ڼ���ʱ�ɷ�Ӧ��д���䷴Ӧ����ʽ������������������������������������
��4)E�ĵ��ʺ���������ϡ�����пɷ�Ӧ�����˽������Ӧ��Ƴ�ԭ��أ���д��������Ӧ����ʽ��������������������������������������������
��5��úȼ�ղ�������������B����������������صĻ������⣬��ˣ�����AH4����ԭ��������Ⱦ����֪��
�� AH4(g)+2 BO2��g)�� B2(g)+AO2(g)+2H2O (g) ��H1����867kJ��mol
�� 2BO2(g) ?B2O4(g) ��H2=��56.9 kJ��mol
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽ������������������������������������������������
��14�֣��±�ΪԪ�����ڱ���һ���֣�
 �� ������ | | | | |||||
| 1 | �� | | | | | | | |
| 2 | | | | | | �� | | |
| 3 | �� | | | �� | | �� | �� | |
��1��д��Ԫ�آ������ڱ��е�λ�ã��������� ������
��2���ڢۢݵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ�������� ������
��3���ܢݢ���̬�⻯����ȶ�����ǿ������˳���������� ����
��4���٢ڢۢ��е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д���������ֻ�
����ĵ���ʽ�������� ��������
������������Ԫ����ɵ����ʼ䣬��һ�������£����Է�����ͼ�еı仯������A��һ
�ֵ���ɫ���塣��
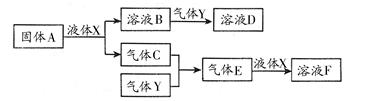
��1��д������A��Һ��X��Ӧ�����ӷ���ʽ�������������� ����������
��2������Y��һ�ִ�����Ⱦ�ֱ���ŷŻ��γ����ꡣ������ҺB���գ���B��Y���ʵ���֮��Ϊ1��1��ǡ����ȫ��Ӧʱ��������ҺD������Ϊ���� �������ѧʽ������֪��ҺD�����ԣ���D��Һ�и�������Ũ���ɴ�С��˳��Ϊ ����
��3����100 mL 18 mol/L��FŨ��Һ�м������ͭƬ������ʹ֮��ַ�Ӧ��������������������£�����Ϊ��������������������
A��40.32 L B��30.24 L C��20.16 L D��13.44 L