��Ŀ����
9��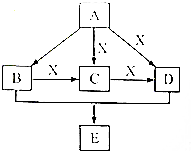 A��B��C��D��E��Ϊ�������л��X��һ�����������A�����壬�����е����ֳ�����ζƷ�зֱ���B��D��E��һ�ֳ������л��ܼ��ͻ���ԭ�ϣ�������2���н��ܴ�A�ϳ�E�ļ��ֿ��ܵĺϳ�·�ߣ���ת����ϵ��������ͼ��ʾ��ͼ�в��ֲ��P��������ȥ�����ش��������⣺
A��B��C��D��E��Ϊ�������л��X��һ�����������A�����壬�����е����ֳ�����ζƷ�зֱ���B��D��E��һ�ֳ������л��ܼ��ͻ���ԭ�ϣ�������2���н��ܴ�A�ϳ�E�ļ��ֿ��ܵĺϳ�·�ߣ���ת����ϵ��������ͼ��ʾ��ͼ�в��ֲ��P��������ȥ�����ش��������⣺��1��д��A�ĵ���ʽ
 ����ҵ�ϴ������A�ķ�����ʯ���ѽ⣮
����ҵ�ϴ������A�ķ�����ʯ���ѽ⣮��2�����й���A��C��˵����ȷ����bc
a��A��C��������ˮ
b��A��C���ܺ�����KMnO4 ��Һ��Ӧ
c��A��C���ܷ����ӳɷ�Ӧ
d�������ʵ�����A��C��ȫȼ��ʱ����O2 ����ͬ��
��3��д��B��D��Ӧ����E�Ļ�ѧ����ʽ��CH3CH2OH+CH3COOH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O����4��E�ж���ͬ���칹�壬���в���������E��ͬ���칹������ʵ���b��
a��������H2������Ӧ���������Ʒ�Ӧ����H2
b.1mol�������������Ʒ�Ӧ����1molH2��
���� ���������ζ�����ԣ��dz��õ���ζ����ʳ����Ҫ�ɷ�Ϊ���ᣬ�Ҵ����д���ζ�����Ƶľƿ������ã��dz��õĵ�ζƷ֮һ�������е����ֳ�����ζƷ�зֱ���B��D������ӦΪCH3COOH��C2H5OH��X��һ������Ҵ�ͨ�������õ���ȩ����ȩ�����õ����ᣬ����X��������BΪ�Ҵ���CΪ��ȩ��DΪ���ᣬ������A�����壬AΪ��ϩ�����õ������ʴ�����֤��
A��B��CH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����ӦΪCH2=CH2+H2O$��_{���ȼ�ѹ}^{����}$CH3CH2OH��
A��D��CH2=CH2��������Ӧ�������ᣬ��ӦΪ��CH2=CH2+O2$��_{���ȼ�ѹ}^{����}$CH3COOH��
B��C���Ҵ���Cu��Ag�����������·���������Ӧ����ӦΪ��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3CHO+2H2O��
C��D��CH3CHO��������Ӧ��ȩ��������ΪCH3COOH����ӦΪ��2CH3CHO+O2$��_{��}^{����}$2CH3COOH��
B+D��E��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O������EΪCH3COOCH2CH3��
CH3COOCH2CH3+H2O������EΪCH3COOCH2CH3��
��1��������ϩ����ʽΪC2H4��������̼ԭ����̼ԭ��ͨ�����Թ��õ��Ӷ������ӣ�̼ԭ������ԭ��ͨ��һ�Թ��õ��Ӷ���������д����ʽ����ҵ�ϴ��������ϩ�ķ�����ʯ���ѽ⣻
��2������A��C�����ʷ�����AΪ��ϩ����ϩ����̼̼˫����CΪ��ȩ������ȩ�������л���Ļ�ѧʽΪCxHyOz��1mol���л������ĵ����������ʵ���Ϊ��x+$\frac{y}{4}$-$\frac{z}{2}$��mol���ֱ���������л�����ȼ�յĺ�������Ȼ����бȽϣ�
��3��BΪ�Ҵ���DΪ���ᣬ���߷���������Ӧ��������������ˮ����Ӧ����Ϊ�����ǻ�������ԭ�ӣ�
��4��EΪ����������ͬ���칹�������ࡢ���ࡢ��ȩ�ࡢ��ͪ��ȣ��ݴ˷�����
��� �⣺���������ζ�����ԣ��dz��õ���ζ����ʳ����Ҫ�ɷ�Ϊ���ᣬ�Ҵ����д���ζ�����Ƶľƿ������ã��dz��õĵ�ζƷ֮һ�������е����ֳ�����ζƷ�зֱ���B��D������ӦΪCH3COOH��C2H5OH��X��һ������Ҵ�ͨ�������õ���ȩ����ȩ�����õ����ᣬ����X��������BΪ�Ҵ���CΪ��ȩ��DΪ���ᣬ������A�����壬AΪ��ϩ�����õ������ʴ�����֤��
A��B��CH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����ӦΪCH2=CH2+H2O$��_{���ȼ�ѹ}^{����}$CH3CH2OH��
A��D��CH2=CH2��������Ӧ�������ᣬ��ӦΪ��CH2=CH2+O2$��_{���ȼ�ѹ}^{����}$CH3COOH��
B��C���Ҵ���Cu��Ag�����������·���������Ӧ����ӦΪ��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3CHO+2H2O��
C��D��CH3CHO��������Ӧ��ȩ��������ΪCH3COOH����ӦΪ��2CH3CHO+O2$��_{��}^{����}$2CH3COOH��
B+D��E��CH3CH2OH��CH3COOH��Ũ���������·�Ӧ��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O������EΪCH3COOCH2CH3��
CH3COOCH2CH3+H2O������EΪCH3COOCH2CH3��
��1����ϩ������̼̼��˫��������̼ʣ��ۼ���H���ͣ��ɴ�д������ʽΪ�� ��
��
�ѽ�������ѻ��Ի�ö�����������Ϊ��Ҫ�ɷֵ�ʯ�ͼӹ����̣�����ҪΪ��ϩ����ϩ������ϩ�Ȳ��������������Թ�ҵ�ϻ�ô�������ϩ����ϩ������ϩ�ķ�����ʯ���ѽ⣬
�ʴ�Ϊ�� ��ʯ���ѽ⣻
��ʯ���ѽ⣻
��2��AΪ��ϩ��CΪ��ȩ��
a����ϩ������ˮ����ȩ������ˮ����a����
b����ϩ̼̼˫���ܱ����Ը��������������ȩȩ���ܱ�����KMnO4 ��Һ��������b��ȷ��
c����ϩ̼̼˫���ܺ����������ʼӳɣ��磺CH2=CH2+H2$��_{��}^{����}$C2H6����ȩȩ���ܺ������ȷ����ӳɷ�Ӧ���磺CH3CHO+H2$��_{��}^{����}$CH3CH2OH����c��ȷ��
d�����л���Ļ�ѧʽΪCxHyOz��1mol���л������ĵ����������ʵ���Ϊ��x+$\frac{y}{4}$-$\frac{z}{2}$��mol�������ʵ�����A��C��ȫȼ��ʱ����O2 ����Ϊ3��2.5����d����
��ѡbc��
��3��B���Ҵ���DΪCH3COOH�����߷���������Ӧ�������ǻ�������ԭ�ӣ���ӦΪ��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3CH2OH+CH3COOH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��4��EΪ����������ͬ���칹�������ࡢ���ࡢ��ȩ�ࡢ��ͪ��ȣ�
a����ȩ�ࡢ��ͪ�������H2������Ӧ���������Ʒ�Ӧ����H2���磺HOCH2CH2CH2CHO��HOCH2COCH2CH3�ȣ���a��ȷ��
b�������Ʒ�Ӧ���������贼�ǻ����Ȼ��������Ƿֱ����Ʒ�Ӧ�Լ��������������ʵ�����Ϊ1��1��$\frac{1}{2}$��1mol�������������Ʒ�Ӧ����1molH2��1mol������2mol���ǻ���2mol�Ȼ�����1mol���ǻ���1mol�Ȼ�����Ȼ��ѧ��ѧ��ѧϰ��ͬ���칹�����ࡢ���ࡢ��ȩ�ࡢ��ͪ��û�з���Ҫ��ģ���b����
��ѡb��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬���չ����������ʵĹ�ϵ������ϩ��������ȩ������֮���ת����ϵ�ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

| A�� | ��ͨ���Cl2Ϊ2.24 Lʱ����Ӧ�е���ת����ĿΪ0.1 NA | |
| B�� | ����Һ��Na+Ϊ0.4 NAʱ����Һ�е�Cl-Ϊ0.2 NA | |
| C�� | ������ת����ĿΪ0.2 NAʱ����Һ��������14.2 g | |
| D�� | ����Һ��������7.1 gʱ����Һ��ClO-Ϊ0.1 NA |
| A�� | 1 mol Cu����������Ӧ��ת�Ƶĵ�����ΪnA | |
| B�� | ��״���£�22.4 L�ȷ��к��еķ�����ΪnA | |
| C�� | 25��ʱ��pH=13��Ba��OH��2��Һ�к��е�OH-��ĿΪ0.1nA | |
| D�� | 53.5g NH4Cl�к��еķ�����ΪNA |
| A�� | �ƹ�ʹ�ú���ϴ�Ӽ� | B�� | ��O3���Cl2������ˮ������ | ||
| C�� | �ù�ҵ��ˮֱ�ӹ��ũ�� | D�� | ��H2SO4�ķ�ˮ��BaCl2�������ŷ� |
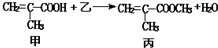 �ж�����˵���в���ȷ���ǣ�������
�ж�����˵���в���ȷ���ǣ�������| A�� | ������Ϊ�״� | |
| B�� | �÷�ӦΪȡ����Ӧ | |
| C�� | �����Ҷ���������Ʒ�Ӧ�������� | |
| D�� | �ס��ҡ���������������Ȼ�̼��Һ�����ӳɷ�Ӧ |
| A�� | X����Ԫ���γɵĻ������п��ܺ��й��ۼ� | |
| B�� | ��������Ԫ�صļ�������Y���ӵİ뾶��С | |
| C�� | Z��W������������������� | |
| D�� | RԪ�غ����������һ��ǿ��W |
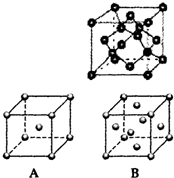 ����A��B��C��D��E��F��Gԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ���������Ԫ����EԪ��ԭ�Ӱ뾶���Aԭ�Ӱ뾶��С��FԪ����GԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����FԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺
����A��B��C��D��E��F��Gԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڣ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ���������Ԫ����EԪ��ԭ�Ӱ뾶���Aԭ�Ӱ뾶��С��FԪ����GԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����FԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���ش��������⣺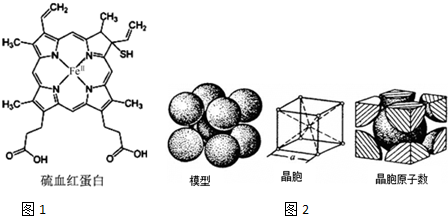
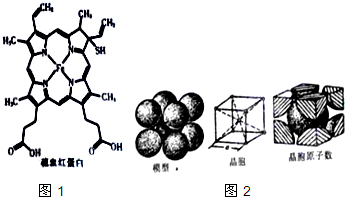 Ѫ�쵰���Ǹߵ��������ڸ�����������һ�ֵ����ʣ���дΪHB��HCB����������Ѫ�쵰����ȣ���Ѫ�쵰�ײ���������ϣ����ʧȥЯ��������������ͼ1��ʾΪ��Ѫ�쵰�Ľṹ����ش��������⣺
Ѫ�쵰���Ǹߵ��������ڸ�����������һ�ֵ����ʣ���дΪHB��HCB����������Ѫ�쵰����ȣ���Ѫ�쵰�ײ���������ϣ����ʧȥЯ��������������ͼ1��ʾΪ��Ѫ�쵰�Ľṹ����ش��������⣺