��Ŀ����
4��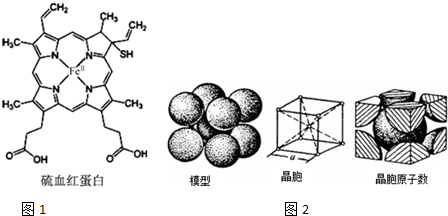
 Ѫ�쵰���Ǹߵ��������ڸ�����������һ�ֵ����ʣ���дΪHB��HCB����������Ѫ�쵰����ȣ���Ѫ�쵰�ײ���������ϣ����ʧȥЯ��������������ͼ1��ʾΪ��Ѫ�쵰�Ľṹ����ش��������⣺
Ѫ�쵰���Ǹߵ��������ڸ�����������һ�ֵ����ʣ���дΪHB��HCB����������Ѫ�쵰����ȣ���Ѫ�쵰�ײ���������ϣ����ʧȥЯ��������������ͼ1��ʾΪ��Ѫ�쵰�Ľṹ����ش��������⣺��1����Ѫ�쵰���������ǽ���Ԫ���е縺�����Ԫ����O����һ����������Ԫ�ص�ԭ����N��
��2�����ݵȵ�����ԭ���ƶ���Ԫ�ص�һ��ͬ��������O3�Ǽ��Է��ӣ�����ԡ��Ǽ��ԡ�����Ũ����ճ�Ƚϴ��ԭ���Ƿ��Ӽ�����γ������
��3����Ѫ�쵰����C��һ�ֵ��ӻ���ʽΪsp2��sp3���ڴ�����á��������N��Fe2+�γɵ���λ����
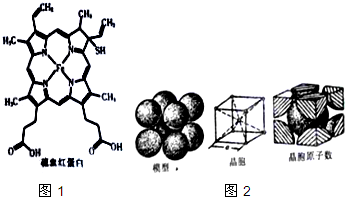
��4����������ܶѻ���ʽΪ���������ṹ���ṹ��ͼ2��ʾ��������ԭ�ӵİ뾶Ϊrpm����ʽ��������öѻ���ʽ�Ŀռ�������68%��
���� ��1��ͬһ����������ҵ縺������ͬһ�������϶��µ縺�Լ�С��ͬ�������϶��£�Ԫ�صĵ�һ�����ܼ�С��ͬһ�����������Ԫ�صĵ�һ�����ܳ��������ƣ�ע��ͬһ���ڵĵڢ�AԪ�صĵ�һ�����ܴ��ڵڢ�A��ģ��ڢ�A��Ĵ��ڵڢ�A��ģ�
��2��������ͬԭ�����������������ķ��ӻ����ӽеȵ����壬�ȵ�����Ľṹ���������ƣ����Ӽ�����γ����ʹ���ʵ�ճ������
��3������Cԭ���γɵĦҼ���Ŀ�ж��ӻ����ͣ�Nԭ���������5�����ӣ�ֻ��Ҫ�γ�3�������ɴﵽ�ȶ��ṹ������ͼ���γ�˫����Nԭ���γ�������������Ȼ��һ��Ϊ��λ����
��4�����þ�̯�����㾧����Feԭ����Ŀ����������Feԭ���������λ����Խ����ϵ�Feԭ�����ڣ��ݴ˼��㾧�����ⳤ���������㾧��������ռ�������=$\frac{ԭ�������}{�������}$��100%��
��� �⣺��1����Ѫ�쵰���������ǽ���Ԫ���У�C��H��N��O��S��ͬ����Ԫ�ش�����Ԫ�صĵ縺�Գ��������ƣ����е縺��C��N��O��H�ĵ縺����С�����Ե縺��������O��
ͬ�������϶��£�Ԫ�صĵ�һ�����ܼ�С��ͬһ�����������Ԫ�صĵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ����������Ԫ�ص�ԭ����N��
�ʴ�Ϊ��O��N��
��2��O3��SO2�ǵȵ����壬SO2����ΪV�ͷ��ӣ�������������IJ��غϣ��Ǽ��Է��ӣ��ȵ�����Ľṹ���ƣ�O3�Ǽ��Է��ӣ���������к����ǻ����γ������Ũ����ճ�Ƚϴ��ԭ���Ƿ��Ӽ�����γ������
�ʴ�Ϊ�����ԣ����Ӽ�����γ������
��3������Cԭ���γ�4���Ҽ�����ȡsp3�ӻ����γ�˫����Cԭ���γ�3���Ҽ�����ȡsp2�ӻ���
Nԭ���������5�����ӣ�ֻ��Ҫ�γ�3�������ɴﵽ�ȶ��ṹ������ͼ���γ�˫����Nԭ���γ�������������Ȼ��һ��Ϊ��λ�����á��������N��Fe2+�γɵ���λ��Ϊ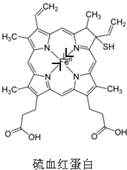 ��
��
�ʴ�Ϊ��sp2��sp3�� ��
��
��4�����������к���Feԭ����ĿΪ1+8��$\frac{1}{8}$=2����ԭ�ӣ���������ԭ�ӵ����Ϊ��2��$\frac{4}{3}$���С�r3�����������У���Խ�����Ϊ������ԭ�����У�����Խ���Ϊ4r�������߳�Ϊ��$\frac{4r}{\sqrt{3}}$���������Ϊ����$\frac{4r}{\sqrt{3}}$r��3���ռ�������Ϊ��[��2��$\frac{4}{3}$���С�r3���£�$\frac{4r}{\sqrt{3}}$r��3]��100%=68%��
�ʴ�Ϊ��68%��
���� ���⿼�����ʽṹ�����ʣ��漰Ԫ�����ʵݱ���ɡ��ȵ���ԭ��Ӧ�á��������ʡ���λ������������ȣ���4���м���Ϊ�״��㡢�ѵ㣬��Ҫѧ���߱�һ������ѧ������������Ŀ�Ѷ��еȣ�

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�| A�� | ���Ӿ�����һ�����й��ۼ� | |
| B�� | ���Ӿ�����һ���������Ӽ� | |
| C�� | ���м��Լ��ķ���һ���Ǽ��Է��� | |
| D�� | ���зǼ��Լ��ķ���һ���ǷǼ��Է��� |
| A | B | C | D |
 |  |  |  |
| Si3N4 | NaOH | Al��OH��3 | C12H22O11 |
| �������������� | �Ʒ��� | ����ҩ | ����ζ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ���³�ѹ�£�1mol��������4nA������ | |
| B�� | 1molNa2O2������CO2��ַ�Ӧת����2nA������ | |
| C�� | ��49gH2SO4��������Һ�к���nA����ԭ�� | |
| D�� | ��״���£�22.4LHCHO����nA������ |
| A�� | ��SO2ˮ��Һ�м�������NaHCO3��ĩ�������ݲ�����˵��SO2ˮ��Һ������ | |
| B�� | ��SO2ˮ��Һ�еμ�Ba��NO3��2��Һ���а�ɫ����������˵��SO2ˮ��Һ�к���SO42- | |
| C�� | ��SO2ˮ��Һ��ͨ��H2S���壬�е���ɫ����������˵��SO2ˮ��Һ���������� | |
| D�� | ��KMnO4��Һ�еμ�SO2ˮ��Һ����Һ��ɫ��ȥ��˵��SO2ˮ��Һ����Ư���� |
 ��Դ�������������������ᷢչ������أ��о����ǵ��ۺ���������Ҫ���壮
��Դ�������������������ᷢչ������أ��о����ǵ��ۺ���������Ҫ���壮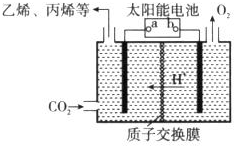
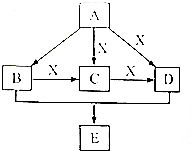 A��B��C��D��E��Ϊ�������л��X��һ�����������A�����壬�����е����ֳ�����ζƷ�зֱ���B��D��E��һ�ֳ������л��ܼ��ͻ���ԭ�ϣ�������2���н��ܴ�A�ϳ�E�ļ��ֿ��ܵĺϳ�·�ߣ���ת����ϵ��������ͼ��ʾ��ͼ�в��ֲ��P��������ȥ�����ش��������⣺
A��B��C��D��E��Ϊ�������л��X��һ�����������A�����壬�����е����ֳ�����ζƷ�зֱ���B��D��E��һ�ֳ������л��ܼ��ͻ���ԭ�ϣ�������2���н��ܴ�A�ϳ�E�ļ��ֿ��ܵĺϳ�·�ߣ���ת����ϵ��������ͼ��ʾ��ͼ�в��ֲ��P��������ȥ�����ش��������⣺ ����ҵ�ϴ������A�ķ�����ʯ���ѽ⣮
����ҵ�ϴ������A�ķ�����ʯ���ѽ⣮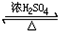 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��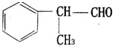 ����һ����Ҫ�Ļ���ԭ�ϣ���ϳ�·�����£�
����һ����Ҫ�Ļ���ԭ�ϣ���ϳ�·�����£�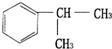 $��_{����}^{��Cl_{2}}$
$��_{����}^{��Cl_{2}}$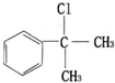 $��_{��}^{��NaOH/��}$A$\stackrel{��HBr/H_{2}O_{2}}{��}$B$��_{��}^{���Լ�X}$C$\stackrel{��������Ӧ}{��}$�⻯����ȩ
$��_{��}^{��NaOH/��}$A$\stackrel{��HBr/H_{2}O_{2}}{��}$B$��_{��}^{���Լ�X}$C$\stackrel{��������Ӧ}{��}$�⻯����ȩ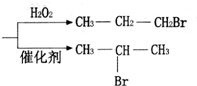
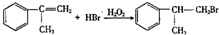 ��
��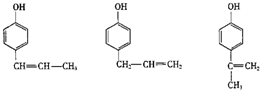 ��������һ�֣�����д��һ�ּ��ɣ���
��������һ�֣�����д��һ�ּ��ɣ���