��Ŀ����
19��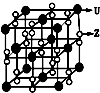 X��Y��Z��UΪԭ���������������ǰ������Ԫ�أ�X��Y��Z����Ԫ��λ��ͬһ���ڣ����л�̬Yԭ�ӵ�2p������ڰ����״̬��Y��Z�ĵ��ʿ���ͨ������Һ̬�����ķ��������Ƶã���XZ2��Y2Z��Ϊ�ȵ����壮��U�Ļ��������ɫΪ��ɫ���Իش��������⣺
X��Y��Z��UΪԭ���������������ǰ������Ԫ�أ�X��Y��Z����Ԫ��λ��ͬһ���ڣ����л�̬Yԭ�ӵ�2p������ڰ����״̬��Y��Z�ĵ��ʿ���ͨ������Һ̬�����ķ��������Ƶã���XZ2��Y2Z��Ϊ�ȵ����壮��U�Ļ��������ɫΪ��ɫ���Իش��������⣺��1��U���ʵ�ԭ�Ӷѻ���ʽΪ���������ѻ���Y�ļ۵����Ų�ʽΪ2s22p3��
��2�����������Z��U��Ԫ����ɣ��侧����ͼ��ʾ��
�ټĻ�ѧʽΪKO2��
�������йظþ����˵������ȷ����BC��ѡ��ѡ����ĸ����
A��ÿ�������к���14��U+��13��Z2-
B��������ÿ��U+��Χ���������U+��6��
C���þ����������Ӿ���
�ۼ��ܶ�Ϊa g•cm-3�����������$\frac{284}{a{N}_{A}}$��10-6 m3��ֻҪ���г�����ʽ�������ӵ�������ֵ��NA��ʾ����
���� X��Y��Z��UΪԭ���������������ǰ������Ԫ�أ���̬Yԭ�ӵ�2p������ڰ����״̬������Χ�Ų�Ϊ2s22p3����YΪ��Ԫ�أ�X��Y��Z����Ԫ��λ��ͬһ���ڣ�Y��Z�ĵ��ʿ���ͨ������Һ̬�����ķ��������Ƶã���XZ2��Y2Z��Ϊ�ȵ����壬X��Z��������֮��Ϊ14����ΪCO2��N2O����XΪ̼Ԫ�ء�ZΪ��Ԫ�أ���U�Ļ��������ɫΪ��ɫ����UΪ��Ԫ�أ��ݴ˽��
��� �⣺X��Y��Z��UΪԭ���������������ǰ������Ԫ�أ���̬Yԭ�ӵ�2p������ڰ����״̬������Χ�Ų�Ϊ2s22p3����YΪ��Ԫ�أ�X��Y��Z����Ԫ��λ��ͬһ���ڣ�Y��Z�ĵ��ʿ���ͨ������Һ̬�����ķ��������Ƶã���XZ2��Y2Z��Ϊ�ȵ����壬X��Z��������֮��Ϊ14����ΪCO2��N2O����XΪ̼Ԫ�ء�ZΪ��Ԫ�أ���U�Ļ��������ɫΪ��ɫ����UΪ��Ԫ�أ�
��1��UΪ��Ԫ�أ�K���ʵ�ԭ�Ӷѻ���ʽΪ���������ѻ���YΪ��Ԫ�أ�Y�ļ۵����Ų�ʽΪ2s22p3��
�ʴ�Ϊ�����������ѻ���2s22p3��
��2�����ɾ����ṹ��֪��������Kԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��Oԭ����ĿΪ4��2��$\frac{1}{4}$+8��$\frac{1}{2}$+8��$\frac{1}{4}$=8����Kԭ����Oԭ����Ŀ֮��Ϊ4��8=1��2���ʼĻ�ѧʽΪKO2��
�ʴ�Ϊ��KO2��
��A���ɢ��м����֪��ÿ�������к���4��K+��4O2-����A����
B���ɾ����ṹ��֪��������ÿ��K+��Χ����K+�����O2-��6������B��ȷ��
C���þ�����K+��4O2-���ɣ��������Ӿ��壬��C��ȷ��
�ʴ�Ϊ��BC��
�۾���������Ϊ$\frac{4��71}{{N}_{A}}$g�����ܶ�Ϊa g•cm-3�����������$\frac{\frac{4��71}{{N}_{A}}}{a}$cm3=$\frac{284}{a{N}_{A}}$��10-6m3��
�ʴ�Ϊ��$\frac{284}{a{N}_{A}}$��10-6��
���� ���⿼��ṹ����λ�ù�ϵ����ѧ���������ṹ�����ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���ؼ���ע�����þ�̯�����о����ļ��㣮

 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�| A�� | C2H4O | B�� | C2H6O | C�� | C3H4O2 | D�� | C3H6O2 |
| A�� | �����ͬ | B�� | ������� | C�� | ��������� | D�� | ԭ������� |
| A�� | ��ˮ����ڴ���������ɫ���ƿ�� | |
| B�� | �����Ʊ�����ú���� | |
| C�� | Ũ���ᱣ������ɫ�Լ�ƿ�� | |
| D�� | ̼���ƹ��屣���ڹ��ƿ�� |
| A�� | ��̿��ȼ���м���KClO3 | |
| B�� | Zn��25%��ϡ���ᷴӦ��ȡH2ʱ������98%��Ũ���� | |
| C�� | ��K2S04��BaCl2����Һ��Ӧʱ������ѹǿ | |
| D�� | Na���Ҵ���Ӧʱ�����Ҵ������� |
| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| B�� | ȼú������ú���ۺ����õ���Ҫ;�� | |
| C�� | ��Ӧ���������������������������Ļ�ѧ��Ӧ�Ƿ��ȷ�Ӧ | |
| D�� | ��ѧ���Ķ��Ѻ��γ��뻯ѧ�仯�е������仯�� |
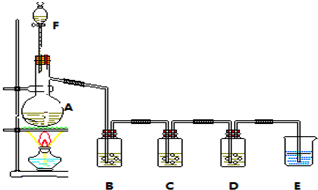
 ������RΪ��������ʵ���������·����ɵõ�DMP��
������RΪ��������ʵ���������·����ɵõ�DMP��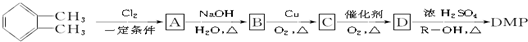
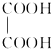 ����һ�������¿������ʵ���1��1������Ӧ���ɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽΪ
����һ�������¿������ʵ���1��1������Ӧ���ɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽΪ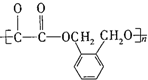 ��
��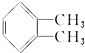 ��Ϊԭ��������������
��Ϊԭ��������������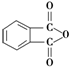 ������ʹ����ij����һ�������·�Ӧ��ȡDMP��������ô���ȡDMP�Ļ�ѧ����ʽΪ
������ʹ����ij����һ�������·�Ӧ��ȡDMP��������ô���ȡDMP�Ļ�ѧ����ʽΪ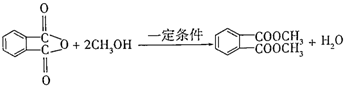 ��
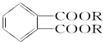
��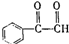 ��BҲ���ڶ���ͬ���칹�壬��������������Bͬ���칹����6�֣�
��BҲ���ڶ���ͬ���칹�壬��������������Bͬ���칹����6�֣�