��Ŀ����
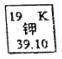
����Ŀ����ͼ��ʾΪԪ�����ڱ��м�Ԫ�ؿ�ͼ��������39.10����ʾ����________��
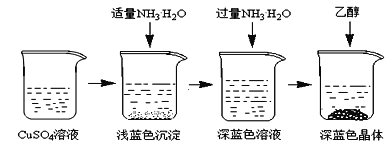
ij��ѧ��ȤС������ȥ16g�����Ȼ����л��е������Ȼ��ƺ�����þ���ʣ����ʵ�鷽�����£���֪������NaCl���ܽ��Ϊ36g/100g![]() O��
O��

��1����ˮ�ܽ�ʱ���ʺϵ�ȡˮ��Ϊ________
A. 20mL B. 45mL C. 75mL D. 100mL
��2��Ϊ�ӿ��ܽ����ʣ��ɲ�ȡ�ķ�����________��________����д2�֣�
��3�����μ���Ĺ����Լ�Ϊ________��________��________��
��4����ҺA�м����������Ϊ________��������Ҳ�ӹ����������õ��Ȼ��ƵĴ���________��������������û������Ӱ��
��5���������������________������II��������________�����ֲ����ж��õ��IJ���������________��
��6�����õ��Ĺ����Ȼ�����ԭ���������е��Ȼ���������ȣ����________������������������С����������������
��7������A���������εĻ�ѧʽΪ___________��
���𰸡���Ԫ�ص����ԭ������ B ���� ���� �������ƣ����Ȼ����� �Ȼ��������������ƣ� ̼���� ���� û�� ���� �����ᾧ ������ ���� BaSO4��CaCO3��BaCO3
��������
Ԫ�����ڱ��ṩ��ԭ��������ʾijԪ�ص����ԭ��������
��1������16g����ȫ��Ϊ�Ȼ��ƣ�����xgˮ��ǡ���γɱ�����Һ������S/100=����/�ܼ����м��㣬�Ӷ��жϳ����ʵ�ȡˮ����
��2������Ӱ�췴Ӧ���ʵ����ؽ��з�����
��3��þ�������������Ƴ�ȥ��������������Ȼ�����ȥ�������ӡ������ı�������̼���Ƴ�ȥ��
��4����ҺA�к����Ȼ��ơ�ʣ���̼���ƺ��������ƣ�Ҫ�õ��������Ȼ��ƣ��������������ᣬ��ȥ̼������Ӻ����������ӣ����������е��Ȼ����ӷ����ʣ���������ӹ����������õ��Ȼ��ƵĴ���Ӱ�죻
��5�����ݳ������ʵķ��뷽�����з�����
��6�������ڳ�ȥ�Ȼ��ơ�����þ����ʱ�������˹������������ơ�̼���ƣ������ᷴӦ�����Ȼ��ƣ��������õ��Ĺ����Ȼ��Ʊ�ԭ���������е��Ȼ�������Ҫ��
��7��þ�������������Ƴ�ȥ������������þ������������������Ȼ�����ȥ���������ᱵ�����������ӡ������ı�������̼���Ƴ�ȥ������̼��ƺ�̼�ᱵ�������ݴ˷��������ε����ࡣ
����Ԫ�����ڱ��м�Ԫ�ؿ�ͼ��֪��������39.10����ʾ���Ǽ�Ԫ�ص����ԭ��������
�ʴ��ǣ���Ԫ�ص����ԭ��������
��1������16g����ȫ��Ϊ�Ȼ��ƣ�����xgˮ��ǡ���γɱ�����Һ������S/100=����/�ܼ���֪��36g/100g=16g/xg��x=44.4g��ˮ�����Ϊ44.4mL�����Լ�ˮ�ܽ�ʱ���ʺϵ�ȡˮ��Ϊ45mL��
�ʴ�ѡB��
��2��Ϊ�ӿ��ܽ����ʣ��ɲ�ȡ�ķ������ò��������衢����ˮ�ܽ⣻
�ʴ��ǣ����衢���ȣ�
��3��þ�������������Ƴ�ȥ��������������Ȼ�����ȥ�������ӡ������ı�������̼���Ƴ�ȥ��
�ʴ��ǣ��������ƣ����Ȼ��������Ȼ��������������ƣ���̼���ƣ�
��4���������˺���ҺA�к����Ȼ�����ʣ���̼�������������ƣ�Ҫ�õ��������Ȼ��ƣ��������������ᣬ��ȥ̼������Ӻ����������ӣ������е��Ȼ����ӷ�����ˣ���ʹ����ӹ���������Ҳ���Գ�ȥ�������õ��Ȼ��ƵĴ���û��Ӱ�죻
�ʴ��ǣ����û�У�
��5����������Һ���룬���Բ��ù��˷�����������������ǹ��ˣ����Ȼ�����Һ�еõ��Ȼ��ƹ��壬���Բ��������ᾧ������II�������������ᾧ�����˺����������о��õ�������������ʱ����������ת��Һ������ã�������ʱ��������������Һ����ֹҺ��ֲ�������ɱŽ���
�ʴ��ǣ����ˣ������ᾧ����������
��6�������ڳ�ȥ�Ȼ��ơ�����þ����ʱ�������˹����������ơ�̼���ƣ������������Ӻ�ʣ���������ơ�̼���������ᷴӦ�����Ȼ��ƣ��������õ��Ĺ����Ȼ��Ʊ�ԭ���������е��Ȼ�������Ҫ�������
�ʴ��ǣ�����
��7��þ�������������Ƴ�ȥ������������þ������������������Ȼ�����ȥ���������ᱵ�����������ӡ������ı�������̼���Ƴ�ȥ������̼��ƺ�̼�ᱵ���������Ծ������˺���A�к��е���Ϊ�����ᱵ��̼��ƺ�̼�ᱵ����ѧʽΪ��BaSO4��CaCO3��BaCO3��
�ʴ��ǣ�BaSO4��CaCO3��BaCO3��

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д� �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�����Ŀ��Ӱ�컯ѧ��Ӧ���ʵ����غܶ࣬ij��ѧС����ʵ��ķ�������̽����
I��̽���һ��
��ѡҩƷ����Ƭ��пƬ��0.5mol/LH2SO4��1.5mol/LH2SO4��18.4mol/LH2SO4
��ͬѧ�о���ʵ�鱨��
ʵ�鲽�� | ���� | ���� |
�ٷֱ�ȡ�������1.5mol/L����������֧�Թ��У� ��_____________________�� | ��Ӧ���ʣ� п>�� | ����������Խ���ã���Ӧ����Խ�� |
(1)��ͬѧʵ�鱨���е�ʵ�鲽���Ϊ__________________________________��
(2)��ͬѧ��ʵ��Ŀ����_______________________________��Ҫ�ó���ȷ��ʵ����ۣ�������Ƶ�ʵ��������__________________��
��ͬѧΪ�˶����о�Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬������ͼ��ʾװ�ý���ʵ�飺

(3)��ͬѧ��ʵ������Ҫ�ⶨ��������_________________________��
(4)��ͬѧ����ѡ��___________mol/L������ɸ�ʵ�飬������_________________��
II��̽�������
��ѡҩƷ��0.1mol/LNa2S2O3��Һ��0.2mol/LNa2S2O3��Һ��0.1mol/LH2SO4��Һ��0.2mol/LH2SO4��Һ��
��֪��Na2S2O3��H2SO4��Na2SO4��S����SO2����H2O
ʵ�� ��� | Na2S2O3���� | H2SO4���� | �¶ȣ����� |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 10 |
�� | 0.2mol/L5mL | 0.2mol/L5mL | 25 |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 25 |
�� | 0.1mol/L5mL | 0.1mol/L5mL | 40 |
(1)����̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��ѡ��ʵ������___________��
(2)����̽��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��ѡ��ʵ������___________��
(3)�ڸ�ʵ������У���Ҫ�۲�ͼ�¼________________�����Ƚϻ�ѧ��Ӧ���ʵĿ�����
(4)Na2S2O3�ڼ�����Һ�пɱ�I2����ΪNa2SO4��д���÷�Ӧ�����ӷ���ʽ___________________��