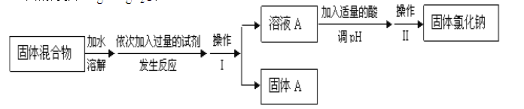
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ”ΑœλΜ·―ßΖ¥”ΠΥΌ¬ ΒΡ“ρΥΊΚήΕύΘ§Ρ≥Μ·―ß–ΓΉι”Ο Β―ιΒΡΖΫΖ®Ϋχ––ΧΫΨΩΓΘ
IΘ°ΧΫΨΩΜνΕ·“ΜΘΚ
±Η―Γ“©ΤΖΘΚΧζΤ§ΓΔ–ΩΤ§ΓΔ0.5mol/LH2SO4ΓΔ1.5mol/LH2SO4ΓΔ18.4mol/LH2SO4
ΦΉΆ§―ß―–ΨΩΒΡ Β―ι±®Ηφ
Β―ι≤Ϋ÷η | œ÷œσ | Ϋα¬έ |
ΔΌΖ÷±π»ΓΒ»ΧεΜΐΒΡ1.5mol/LΒΡΝρΥα”ΎΝΫ÷ß ‘Ιή÷–ΘΜ ΔΎ_____________________ΓΘ | Ζ¥”ΠΥΌ¬ ΘΚ –Ω>Χζ | Ϋπ τΒΡ–‘÷ ‘ΫΜνΤΟΘ§Ζ¥”ΠΥΌ¬ ‘ΫΩλ |
(1)ΦΉΆ§―ß Β―ι±®Ηφ÷–ΒΡ Β―ι≤Ϋ÷ηΔΎΈΣ__________________________________ΓΘ
(2)ΦΉΆ§―ßΒΡ Β―ιΡΩΒΡ «_______________________________ΘΜ“ΣΒΟ≥ω’ΐ»ΖΒΡ Β―ιΫα¬έΘ§ΜΙ–ηΩΊ÷ΤΒΡ Β―ιΧθΦΰ «__________________ΓΘ
““Ά§―ßΈΣΝΥΕ®ΝΩ―–ΨΩ≈®Ε»Ε‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§άϊ”Ο»γΆΦΥυ ΨΉΑ÷ΟΫχ–– Β―ιΘΚ
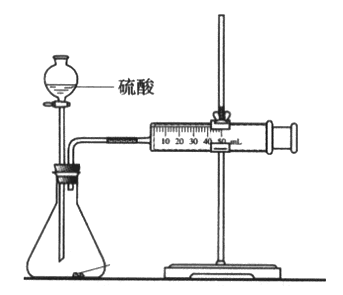
(3)““Ά§―ß‘Ύ Β―ι÷––η“Σ≤βΕ®ΒΡ ΐΨί «_________________________ΓΘ
(4)““Ά§―ß≤ΜΜα―Γ”Ο___________mol/LΝρΥαΆξ≥…ΗΟ Β―ιΘ§άμ”… «_________________ΓΘ
IIΘ°ΧΫΨΩΜνΕ·ΕΰΘΚ
±Η―Γ“©ΤΖΘΚ0.1mol/LNa2S2O3»ή“ΚΓΔ0.2mol/LNa2S2O3»ή“ΚΓΔ0.1mol/LH2SO4»ή“ΚΓΔ0.2mol/LH2SO4»ή“ΚΓΘ
“―÷ΣΘΚNa2S2O3ΘΪH2SO4ΘΫNa2SO4ΘΪSΓΐΘΪSO2ΓϋΘΪH2O
Β―ι ±ύΚ≈ | Na2S2O3”ΟΝΩ | H2SO4”ΟΝΩ | Έ¬Ε»Θ®ΓφΘ© |
ΔΌ | 0.1mol/L5mL | 0.1mol/L5mL | 10 |
ΔΎ | 0.2mol/L5mL | 0.2mol/L5mL | 25 |
Δέ | 0.1mol/L5mL | 0.1mol/L5mL | 25 |
Δή | 0.1mol/L5mL | 0.1mol/L5mL | 40 |
(1)»τœκΧΫΨΩΈ¬Ε»Ε‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§Ω…―ΓΒΡ Β―ι±ύΚ≈”–___________ΓΘ
(2)»τœκΧΫΨΩ≈®Ε»Ε‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§Ω…―ΓΒΡ Β―ι±ύΚ≈”–___________ΓΘ
(3)‘ΎΗΟ Β―ιΙΐ≥Χ÷–Θ§–η“ΣΙέ≤λΚΆΦ«¬Φ________________Θ§ά¥±»ΫœΜ·―ßΖ¥”ΠΥΌ¬ ΒΡΩλ¬ΐΓΘ
(4)Na2S2O3‘ΎΦν–‘»ή“Κ÷–Ω…±ΜI2―θΜ·ΈΣNa2SO4Θ§–¥≥ωΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ___________________ΓΘ
ΓΨ¥πΑΗΓΩΖ÷±πΆΕ»κ¥σ–ΓΓΔ–ΈΉ¥œύΆ§ΒΡΧζΓΔ–Ω ―–ΨΩΫπ τ±Ψ…μΒΡ–‘÷ ”κΖ¥”ΠΥΌ¬ ΒΡΙΊœΒ Έ¬Ε»œύΆ§ “ΜΕ® ±ΦδΡΎ≤ζ…ζΤχΧεΒΡΧεΜΐΘ®Μρ≤ζ…ζ“ΜΕ®ΧεΜΐΒΡΤχΧεΥυ–ηΒΡ ±ΦδΘ© 18.4 ≥ΘΈ¬œ¬Θ§–Ω”κ18.4mol/LH2SO4Ζ¥”Π≤ζ…ζSO2, ΙΕ‘±» Β―ιΒΡΖ¥”Π‘≠άμ≤ΜΆ§ΓΘ 18.4mol/LH2SO4 ΙΧζΕέΜ· ΔΌΓΔΔήΜρΔΌΓΔΔέΜρΔΌΓΔΔέΓΔΔή ΔΎΓΔΔέ …ζ≥…Β»÷ ΝΩΒΡ≥ΝΒμΥυ–ηΒΡ ±Φδ S2O32-+10OH-+4I2=2SO42-+8I-+5H2O
ΓΨΫβΈωΓΩ
IΘ°Θ®1Θ©ΦΉΆ§―ß±μ÷– Β―ι≤Ϋ÷ηΔΎΈΣœρΒ»ΧεΜΐ≈®Ε»Ζ÷±π «0.5 mol/LΓΔ2 mol/LΒΡΝρΥα÷–Φ”»κΆΕ»κ¥σ–ΓΓΔ–ΈΉ¥œύΆ§ΒΡΧζΓΔ–ΩΘΜΖΔœ÷ΘΚΖ¥”ΠΥΌ¬ ΘΚ–Ω>ΧζΘ§ΥΒΟςΫπ τΒΡ–‘÷ ‘ΫΜνΤΟΘ§Ζ¥”ΠΥΌ¬ ‘ΫΩλΘΜ
Θ®2Θ©ΦΉΆ§―ßΒΡ Β―ιΡΩΒΡ «―–ΨΩΫπ τ±Ψ…μΒΡ–‘÷ ”κΖ¥”ΠΥΌ¬ ΒΡΙΊœΒΘΜ“ΣΒΟ≥ω’ΐ»ΖΒΡ Β―ιΫα¬έΘ§ΜΙ–ηΩΊ÷ΤΒΡ Β―ιΧθΦΰ «Ζ¥”ΠΈ¬Ε»”ΠΗΟœύΆ§Θ§’β―υ≤≈Ρή–Έ≥…Ε‘’’ Β―ιΘΜ
Θ®3Θ©““Ά§―ß‘Ύ Β―ι÷–”ΠΗΟ≤βΕ®ΒΡ ΐΨί «“ΜΕ® ±ΦδΡΎ≤ζ…ζΤχΧεΒΡΧεΜΐΘ®Μρ≤ζ…ζ“ΜΕ®ΧεΜΐΒΡΤχΧεΥυ–ηΒΡ ±ΦδΘ©ΘΜ»τœύΆ§ΒΡ ±ΦδΡΎ≤ζ…ζΒΡΤχΧεΧεΜΐ‘Ϋ¥σΘ§‘ρΖ¥”ΠΥΌ¬ ‘ΫΩλΘΜΜρ’Ώ ’Φ·œύΆ§ΧεΜΐΒΡΤχΧεΘ§–η“ΣΒΡ ±Φδ‘ΫΕΧΘ§‘ρΖ¥”ΠΥΌ¬ ‘ΫΩλΘΜ
Θ®4Θ©““Ά§―ß≤ΜΜα―Γ”Ο18.4 mol/LΝρΥαΆξ≥…ΗΟ Β―ιΘ§ «“ρΈΣFe‘Ύ≈®ΝρΥα÷–ΜαΖΔ…ζΕέΜ·œ÷œσΘΜ
IIΘ°(1)»τ“ΣΧΫΨΩΈ¬Ε»Ε‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§”ΠΗΟ «÷ΜΗΡ±δΈ¬Ε»Θ§ΕχΤδΥϊΧθΦΰ≤Μ±δΓΘΩ…―Γ‘ώ Β―ιΉιΚœΈΣΔΌΓΔΔήΜρΔΌΓΔΔέΜρΔΌΓΔΔέΓΔΔήΘΜ
(2) »τœκΧΫΨΩ≈®Ε»Ε‘Μ·―ßΖ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§”ΠΗΟ «÷ΜΗΡ±δ≈®Ε»Θ§ΕχΤδΥϊΧθΦΰ≤Μ±δΓΘΩ…―Γ‘ώ Β―ιΉιΚœΈΣΔΎΓΔΔέΘΜ
(3)‘ΎΗΟ Β―ιΙΐ≥Χ÷–Θ§–η“ΣΙέ≤λΚΆΦ«¬Φ…ζ≥…Β»÷ ΝΩΒΡ≥ΝΒμΥυ–ηΒΡ ±ΦδΘ§ά¥±»ΫœΜ·―ßΖ¥”ΠΥΌ¬ ΒΡΩλ¬ΐΘΜ
(4)Na2S2O3‘ΎΦν–‘»ή“Κ÷–Ω…±ΜI2―θΜ·ΈΣNa2SO4Θ§±Ψ…μ±ΜΜΙ‘≠ΈΣI-Θ§Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣS2O32-+10OH-+4I2=2SO42-+8I-+5H2OΓΘ

ΓΨΧβΡΩΓΩœ¬Ν– Β―ιΖΫΑΗΡή¥οΒΫœύ”Π Β―ιΡΩΒΡΒΡ «
―Γœν | Β―ιΡΩΒΡ | Β―ιΖΫΑΗ |
A | Φλ―ι’αΧ«Υ°Ϋβ…ζ≥…ΤœΧ―Χ« | »Γ ΝΩ’αΧ«»ή”Ύ Δ”–’τΝσΥ°ΒΡ ‘Ιή÷–Θ§ΒΈ»κœΓΝρΥαΦ”»»“ΜΕΈ ±ΦδΘ§ά以ȧ¸»κ–¬÷Τ«β―θΜ·Ά≠–ϋΉ«“ΚΘ§Φ”»»÷ΝΖ–ΧΎΘ§Ιέ≤λ”–ΈόΉ©Κλ…Ϊ≥ΝΒμ |
B | Β―ι “÷Τ±Η«β―θΜ·ΧζΫΚΧε | œρ Δ”–25mL’τΝσΥ°ΒΡ…’±≠÷–ΒΈ»κ5-6ΒΈ¬»Μ·Χζ±ΞΚΆ»ή“ΚΘ§Φ”»»÷σΖ–÷Ν»ή“Κ≥ ΚλΚ÷…ΪΘ§ΆΘ÷ΙΦ”»» |
C | ±»ΫœAgClΓΔAgIΒΡKsp¥σ–Γ | œρ Δ”–10ΒΈ0.1mol/LAgNO3 »ή“ΚΒΡ ‘Ιή÷–ΒΈΦ”0.1mol/LNaCl»ή“Κ÷Ν≤Μ‘Ό”–≥ΝΒμ…ζ≥…Θ§‘ΌΒΈΦ”0.1mol/lKI»ή“Κ |
D | ±»ΫœMgΓΔAlΒΡΫπ τ–‘«Ω»θ | ”ΟΒΦœΏΝ§Ϋ”ΟΨΚΆ¬ΝΤ§Θ§≤ε»κ Δ”–«β―θΜ·ΡΤ»ή“ΚΒΡ…’±≠÷–Θ§Ιέ≤λΤχ≈ί |
A. A B. B C. C D. D