��Ŀ����
����Ŀ�������İ���ͭ����([Cu(NH3)4]SO4��H2O)������ɱ�����ýȾ�����ڼ��Զ�ͭ��Ҳ���������Һ����Ҫ�ɷ֣��ڹ�ҵ����;�㷺�������¸������ڿ����в��ȶ�������ʱ�����ֽ⡣ij��ѧ��ȤС����Cu�ۡ�3mol/L�����ᡢŨ��ˮ��10% NaOH��Һ��95%���Ҵ���Һ��0.500 mol/Lϡ���ᡢ0.500 mol/L��NaOH��Һ���ϳ������İ���ͭ���岢�ⶨ�䴿�ȡ�
I��CuSO4��Һ���Ʊ�
�ٳ�ȡ4gͭ�ۣ���A����������10���Ӳ����Ͻ��裬������ȴ��
�����������м���30mL 3mol/L�����ᣬ��A�й��������������У����Ȳ����Ͻ��衣
�۳��ȹ��˵���ɫ��Һ��
(1)A����������Ϊ________________________________��
(2)ijͬѧ��ʵ������1.5g��ͭ��ʣ�࣬��ͬѧ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���Խ�����ԭ��__________________________________________��
II��������Ʊ�
�������Ʊ���CuSO4��Һ����ͼ��ʾ���в���
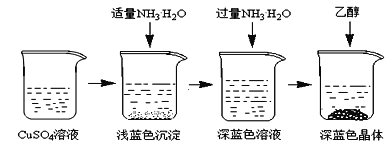
(3)��֪dz��ɫ�����ijɷ�ΪCu2(OH)2SO4����д�����ɴ˳��������ӷ�Ӧ����ʽ___________________��
(4)��������ʱ���ü����Ҵ��ķ�����������Ũ���ᾧ��ԭ����________________________��
III���������IJⶨ
��ȷ��ȡwg������������ˮ�ܽ���ע����ͼ��ʾ������ƿ����Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������������ˮ��ϴ�����ڱڣ���V1mL0.5mol/L���������Һ��ȫ���ա�ȡ�½���ƿ����0.5mol/L NaOH����Һ�ζ���ʣ��HCl(ѡ�ü�����ָʾ��)�����յ�ʱ����V2mLNaOH��Һ��
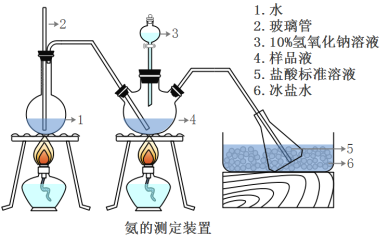
(5)Aװ���г������ܵ�����_________________����Ʒ�а������������ı���ʽ_______��
(6)����ʵ���������ʹ�������ⶨ���ƫ�ߵ�ԭ����____________________��
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܡ�
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӡ�
C���ζ�������ѡ�÷�̪��ָʾ����
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڡ�
���𰸡����� ��Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4 2Cu2++2NH3
5H2Oʧˮ���CuSO4 2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵���
H2O+SO42-=Cu2(OH)2SO4+2NH4+ �����İ���ͭ�����������ȷֽ� ƽ����ѹ����ֹ�����͵��� ![]() BD
BD
��������
(1) ��������һ���������н�����
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4�������ݴ�д�����ӷ���ʽ��
(4) ������狀�ͭ������Ʊ������У������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
(5) ��A��ѹ������ʱ����������Һ��������ʹAƿ��ѹ���ȶ���
���ݵζ���ȥ���������Ƶ����ʵ������Լ�����백��Ӧ����������ʵ�����������������İ��������ʵ������ټ����NH3�������ٷ�����
(6) ������Ʒ�а���������������ʽ![]() ������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
������������V2ƫС����ʹ�������ⶨ���ƫ�ߣ��ݴ˷�����
(1) ��������һ���������н��У���ͭ��ת��ΪCuO��
�ʴ�Ϊ��������
(2) ���Ƶõ�CuSO4��Һ������һ�������м���Ũ�����о�Ĥ���֣���ȴ�����ľ����к��а�ɫ��ĩ���ð�ɫ��ĩΪCuSO4��ԭ���Ƿ�Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
�ʴ�Ϊ����Ӧ�������������Ũ�������У�ϡ������Ũ��Ũ�������ˮ��ʹCuSO4![]() 5H2Oʧˮ���CuSO4��
5H2Oʧˮ���CuSO4��
(3)�ɲ������̿�֪������ͭ��Һ��������ˮ��Ӧ����Cu2(OH)2SO4���������ӷ�Ӧ����ʽΪ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
�ʴ�Ϊ��2Cu2++2NH3![]() H2O+SO42-=Cu2(OH)2SO4+2NH4+��
H2O+SO42-=Cu2(OH)2SO4+2NH4+��
(4) �������Ϣ��֪�������İ���ͭ�����������ȷֽ⣬�������Ҵ����Խ��������İ���ͭ������ܽ�ȣ������ھ���������
�ʴ�Ϊ�������İ���ͭ�����������ȷֽ���
(5) ��A��ѹ������ʱ������������Һ��������ʹAƿ��ѹ���ȶ������������ܵ�������ƽ����ѹ����ֹ�����͵�����
�ʴ�Ϊ��ƽ����ѹ����ֹ�����͵�����
��������ݿ�֪�������İ��������ʵ���Ϊ(0.5V1-0.5V2)��10-3mol��
������Ʒ�а�����������Ϊ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
(6)������Ʒ�а���������������ʽ![]() ����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
����ʵ���������V2ƫС����ʹ�������ⶨ���ƫ�ߣ�
A���ζ�ʱδ��NaOH����Һ��ϴ�ζ��ܣ�����������ҺŨ��ƫС�����ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
B������ʱ���ζ�ǰƽ�ӣ��ζ����ӣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⣻
C���ζ�������ѡ�÷�̪��ָʾ�������ĵ����V2ƫ����ʹ�������ⶨ���ƫ�ͣ����������⣻
D��ȡ�½���ƿǰ��δ������ˮ��ϴ�������ƿ�еĵ�����ڣ����ĵ����V2ƫС����ʹ�������ⶨ���ƫ�ߣ��������⡣
�ʴ�Ϊ��BD��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�����Ŀ��������Ϊ�����Դ���Ź㷺��Ӧ��ǰ����������Ȼ���Ʊ��������������¡�

��ش��������⣺
I��ת��������Ȼ��ѹ����������30��ʱ����T��F�������£����Ի������������ʾ��ͼ���¡�
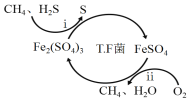
(1)����i�����ӷ�Ӧ����ʽΪ_________________________________________��
(2)��֪��
��Fe3+��pH=l.9ʱ��ʼ������pH=3.2ʱ������ȫ��
��30��ʱ����T.F�������£���ͬpH��FeSO4��Һ��Fe2+�������������±���
pH | 0.7 | 1.1 | 1.5 | 1.9 | 2.3 | 2.7 |
Fe2+����������/g��L-1��h-1 | 4.5 | 5.3 | 6.2 | 6.8 | 7.0 | 6.6 |
��ת�������У������ϱ���ѡ�����pH��Χ��_______<pH<_______������ѡ���ԭ���ǣ�_______________________________________________��
������ת�����ڴ����������£�ˮ������CH4�����������ͼ�ش����⡣
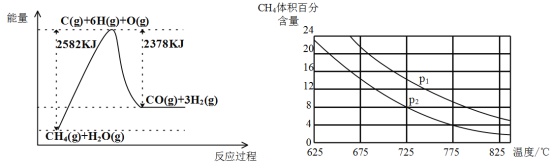
(3)�ٸù��̵��Ȼ�ѧ����ʽ��__________________________________________��
�ڱȽ�ѹǿP1��p2�Ĵ�С��ϵ��P1 _________ P2(ѡ����>����<������=��)��
����һ���¶Ⱥ�һ��ѹǿ�µ�����ɱ���ܱ������г���1molCH4��1mol��ˮ������ַ�Ӧ��ƽ������ʼʱ��������ܶ���ƽ��ʱ������ܶȵ�1.4��������ʱ���������Ϊ2L,��÷�Ӧ��ƽ�ⳣ��Ϊ______________(�������2λ��Ч����)��
����CO�任��500��ʱ��CO��һ����ˮ��Ӧ����CO2��H2��
����H2�ᴿ����CO2��H2����õ�H2�Ĺ�����ʾ��ͼ
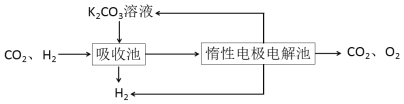
(4)���ճ��з�����Ӧ�����ӷ���ʽ��____________________________________��
����Ŀ��ǰ�����ڵ�A��B��C��D����Ԫ�������ڱ��о���Ԫ��X�������ڡ���֪Ԫ��X���������Ļ�ѧʽΪX2O5��B��Dͬ������BԪ�ص�ԭ�Ӱ뾶��ͬ��Ԫ������С�ģ�C������������Ӧ��ˮ������ǿ�ᡣ
��1��DԪ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽΪ____________________��
��2��A��C��X����Ԫ��ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________________������Ӧ��Ԫ�ط������𣩡�
��3��B��X��D�⻯��ķе��ɸߵ��͵�˳��Ϊ_______________������Ӧ�Ļ�ѧʽ���𣩡�
��4��CԪ�ص�ԭ�ӿ��γɶ������ӣ����Ʋ������������幹�ͣ�CΪ��ĸ������̼Ԫ�أ���
�� | CO32- | CO42- |
���幹������ | _______________ | _______________ |
��5��Ԫ��B��һ���⻯��B2H4������Ҫ����;���й�B2H4��˵����ȷ����_______��
A��B2H4���Ӽ���γ���� B��Bԭ����sp3�ӻ�
C��B2H4�����к���5���Ҽ���1���м� D��B2H4�����ΪҺ̬ʱ�ƻ����ۼ�
��6��EԪ�غ�DԪ����ͬһ���ڣ�����VIII�壬�۲������������ӣ�E(OH)2Ϊ�������������Ũ��ǿ����Һ�п��γ�E(OH)42-��д��E(OH)2��ʽ����ĵ��뷽��ʽ___________________��
��7��FԪ�ػ�̬ԭ��M������5�ԳɶԵ��ӣ�F�γɵĵ����Цġ��á�������ͬ�������壬���־�������ͼ��ʾ����Fԭ�ӵ���λ��֮��Ϊ___________���ġ��á������־����ı߳�֮��Ϊ_____________��