��Ŀ����
5��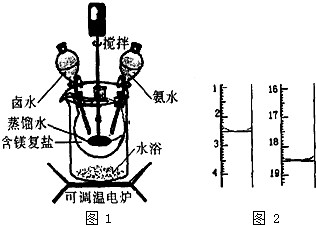 ��ʽ̼��þ�ܶ�С��������Ʒ���������ϣ����ø���MgCO3•��NH4��2CO3•H2O��ԭ���Ƹ���ȡһ�����ĺ�þ���η�������ƿ�У�����������ƿ���ں���ˮԡ���м��ȣ���ͼ1��ʾ������һ����Һ�̱ȼ�������ˮ������������ͬʱ����Ԥ���İ�ˮ�����¶ȵ���40��ʱ��ʼ�Ƚ⣬��ʱ�μ�±ˮ���Ȼ�þ��Һ�����������백ˮ������10minʱ������ϴ�ӣ��˳��Ĺ�����120����¶������¸���õ���ʽ̼��þ��Ʒ��
��ʽ̼��þ�ܶ�С��������Ʒ���������ϣ����ø���MgCO3•��NH4��2CO3•H2O��ԭ���Ƹ���ȡһ�����ĺ�þ���η�������ƿ�У�����������ƿ���ں���ˮԡ���м��ȣ���ͼ1��ʾ������һ����Һ�̱ȼ�������ˮ������������ͬʱ����Ԥ���İ�ˮ�����¶ȵ���40��ʱ��ʼ�Ƚ⣬��ʱ�μ�±ˮ���Ȼ�þ��Һ�����������백ˮ������10minʱ������ϴ�ӣ��˳��Ĺ�����120����¶������¸���õ���ʽ̼��þ��Ʒ����1����ʵ��ѡ��ˮԡ���ȷ�ʽ�����ŵ���ʹ��Ӧ�����Ⱦ��ȣ��¶������ƣ�
��2��40��ʱ���ο�ʼ�Ƚ�����MgCO3•3H2O����������������÷�Ӧ�Ļ�ѧ����ʽΪMgCO3•��NH4��2CO3•H2O+H2O$\frac{\underline{\;\;��\;\;}}{\;}$MgCO3•3H2O+2NH3��+CO2����
��3����ʽ̼��þ��Ʒ��þ����������[w��Mg��%]Խ�ߣ���Ʒ����Խ�ã��ȵ���������Խ�ߣ���Ʒ����Խ�ij����С���ó����ζ���������Ʒ��C1-�ĺ�������ȡ6.1000g��Ʒ�����������ܽ⣬��ϡ�͵Ȳ����������500mL����Һ���������ƹ����б���Ҫ�õ���һ�ּ��������ǵ�����ƽ�������ƽ��ȷ��ȡ25.00mL����Һ������������Һ���еζ����ζ�ǰ��ζ����е�Һ�������ͼ2��ʾ����ζ����������ı�Һ�����Ϊ16mL��
����֪ij�¶�ʱ��һЩ���ε���ɫ��Ksp���±���
| ������ | AgCl | AgBr | AgI | Ag2CrO4 |
| Ksp | 2��10-10 | 5.4��10-13 | 8.3��10-17 | 2��10-12 |
| ��ɫ | ��ɫ | ����ɫ | ��ɫ | ש��ɫ |
a��CaCl2b��NaBr c��NaI d��K2CrO4
�۵ζ�ʱ��Ӧ����Һ�������ԣ�������ǿ���Ի�ǿ���ԣ����в�����ǿ���Ե�ԭ���Ǽ��������£�����������������������������������
��4����֪�Ƶõļ�ʽ̼��þ�ɱ�ʾΪxMgC03��yMg��OH��2H2O������ʽ̼��þ������ag�������������ʣ���������պ��ʣ����������Ϊbg�������Ķ�����̼��������ڱ�״����ΪcL�����ʽ̼��þ��x��y=40c����22.4b-40c��������b��c��ʾ�����軯��
���� ��1��ˮԡ����ʹ���Ⱦ��ȣ��¶������ƣ�
��2������Ԫ���غ�Ͳ����������д����ѧ����ʽ��
��3����ȷ��ȡ6.1000g��ƷҪ�õļ��������ǵ�����ƽ�������ƽ������ͼ2��֪��������Һ�������
�ڵζ�ʵ�������ó�����ɫָʾ��Ӧ������ȫ�����Ȼ����պó�����ɣ��ٵμ���������Һ����ָʾ���������ɲ�ͬ�����������жϣ��ⶨˮ�����Ȼ���ĺ���������ʹ��������ȫ���ɰ�ɫ������ָʾ������Ӧ��ȫ���Լ��ܽ���һ��С���Ȼ��������Ա���Ӧ��Ag+��Cl-����AgCl�����������ɲ�ͬ��ɫ����ָʾ�����յ㣮��ָʾ�����ܽ��Ӧ��AgCl���������жϣ�
�ۼ��������£�������������������������������Ӱ������ζ���
��4������xMgC03��yMg��OH��2H2O$\frac{\underline{\;\;��\;\;}}{\;}$��x+y��MgO+xC02��+��1+y��H2O��������Ʒ����������þ��������������̼������б���ʽ���x��y��
��� �⣺��1��ˮԡ����ʹ��Ӧ�����Ⱦ��ȣ��¶������ƣ�
�ʴ�Ϊ��ʹ��Ӧ�����Ⱦ��ȣ��¶������ƣ�
��2������Ԫ���غ��֪�����ο�ʼ�Ƚ�����MgCO3•3H2O���������������������ӦΪ������̼�Ͱ��������Է�Ӧ�Ļ�ѧ����ʽΪMgCO3•��NH4��2CO3•H2O+H2O$\frac{\underline{\;\;��\;\;}}{\;}$MgCO3•3H2O+2NH3��+CO2����
�ʴ�Ϊ��MgCO3•��NH4��2CO3•H2O+H2O$\frac{\underline{\;\;��\;\;}}{\;}$MgCO3•3H2O+2NH3��+CO2����
��3����ȷ��ȡ6.1000g��ƷҪ�õļ��������ǵ�����ƽ�������ƽ������ͼ2��֪��������Һ�����Ϊ18.5mL-2.5mL=16mL��
�ʴ�Ϊ��������ƽ�������ƽ��16��
��ָʾ����������ָʾ��������ǡ�ó�����ȫ�������������ȳ�����������ȫ���ٵ�����������Һ������һ����ɫ��ͬ�ij�����ָʾ�����յ㣬�Ȼ������廯�����⻯������ɶ���1��1������Ksp����ֱ�ӱȽ��ܽ��Դ�СΪ�Ȼ������廯�����⻯������Ksp�ļ�С��Ӧ�ȳ��ֵ⻯���������ٳ����廯�������ų����Ȼ���������a��b��c�����ϵζ�ʵ���Ŀ�ĺ����ã������������������Ӹ�����Ϊ2��1�����Լ�����ͬŨ�ȵ������ӳ��������ӡ������������Ҫ��Ũ�ȣ�����Ksp����õ���Ksp��AgCl��=[Ag+][Cl-]=2��10-10��Ksp��Ag2CrO4��=[Ag+]2[CrO42-]=2.0��10-12��[Cl-]=$\sqrt{2��1{0}^{-10}}$��[CrO42-]=$\root{3}{\frac{2.0��1{0}^{-12}}{4}}$������ͬŨ�ȵ���������Ҫ��������Ũ��С�ڸ��������Ũ�ȣ�˵���������ܽ�ȴ����Ȼ���������K2CrO4����ָʾ��������ȷ�IJⶨ�Ȼ���ĺ�������d��ȷ��
�ʴ�Ϊ��d��
�ۼ��������£�����������������������������������Ӱ������ζ������Եζ�ʱ����Һ������ǿ���ԣ�
�ʴ�Ϊ�����������£�����������������������������������
��4������xMgC03��yMg��OH��2H2O$\frac{\underline{\;\;��\;\;}}{\;}$��x+y��MgO+xC02��+��1+y��H2O��
40��x+y�� 22.4x
b c
��$\frac{40��x+y��}{b}=\frac{22.4x}{c}$������x��y=40c����22.4b-40c����
�ʴ�Ϊ��40c����22.4b-40c����
���� ������Ҫ�������ʵ����ʵ�ʵ�鷽�����̵�̽�����������ʵĻ�ѧ���ʣ�Ҫ�������������Ҫ����ʵ����Ƶ�ÿһ�������е�ʵ��Ŀ�ģ���Ҫ��ǿ�����ȥ�������ӵķ������Ӷ�ͻ���ѵ㣮�йصļ���Ҫȷ����Ŀ�Ѷ��еȣ�

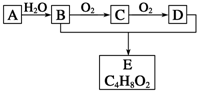 �л�������Aֻ��C��H����Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ��A��B��C��D��E����ͼ��ʾ�Ĺ�ϵ���������ƶ���ȷ���ǣ�������
�л�������Aֻ��C��H����Ԫ���������ʹ��ˮ��ɫ�������������������һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ��A��B��C��D��E����ͼ��ʾ�Ĺ�ϵ���������ƶ���ȷ���ǣ�������| A�� | ����A�ͼ����ѡ�����Ը��������Һ | |
| B�� | B��������Ʒ�ӦD���� | |
| C�� | ����C��������Ӧ�����Ҵ����ǻ�ԭ��Ӧ | |
| D�� | Eû��ͬ���칹�� |
| A�� | ̼��������ҽ���Ͽ���������θ����� | |
| B�� | �ƺͼصĺϽ�������Һ̬�������ڿ����ӷ�Ӧ�����Ƚ����� | |
| C�� | ̼���ƿ����ڲ�������������ֽ����֯�ȹ�ҵ | |
| D�� | �����ƿ�����Ư�� |
| A�� | ˮ | B�� | CuSO4���� | C�� | ��Ƭ | D�� | ͭƬ |
| A�� | 18g D2O��18g H2O�к��е���������Ϊ10NA | |
| B�� | 2L 0.5 mol•L-1��������Һ�к��е�H+������Ϊ2NA | |
| C�� | ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.2NA | |
| D�� | �ܱ�������2mol NO��1mol O2��ַ�Ӧ������ķ�����Ϊ2NA |
| A�� | ij���ȷ�Ӧ���Է����У���˸÷�Ӧ��������Ӧ | |
| B�� | ����Ũ�Ȼ�ʹ�ô��������������Ӱٷ���������ѧ��Ӧ���� | |
| C�� | ��ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں��� | |
| D�� | ���ļ۵��ӵ����Ų�ʽΪ3d54s1������3d44s2����Ϊ�����ȶ� |
| ѡ�� | ���� | ��; | ���� |
| A | H2O2 | ʳƷƯ�� | H2O2����ǿ�����ԣ���Ư��ʳƷ |
| B | SiO2 | �����оƬ | SiO2��һ�����õİ뵼�� |
| C | Fe3O4 | ��ɫͿ�� | Fe3O4��һ�ֺ�ɫ������ |
| D | ŨH2SO4 | ����� | ŨH2SO4����ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |