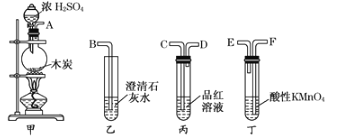
��Ŀ����
����Ŀ��������������Ni2O3����һ����Ҫ�ĵ���Ԫ�����Ϻ����ز��ϡ���ҵ�����ú������ϣ����������ơ�þ�Ͻ�Ϊ������ȡ��������NiC2O4��2H2O�����ٸ������ղ�������ȡ��������������֪����ĸơ�þ�����ξ�������ˮ����������ͼ��ͼ��ʾ��
��ش��������⣺
��1��������Ϊ__________________________��
��2��������H2O2��������Ҫ��Ӧ�����ӷ���ʽΪ_____________________________��
������̼������ҺĿ���ǵ���Һ��pHֵ��4.0��5.0���û�ѧ����ش����̼���Ƶ�Ŀ��_________________________________________________________________��
��3����������NiC2O4��2H2O�����ȿ����и�����ˮ���ڸ��������գ����Ƶ�Ni2O3��ͬʱ��û�����壬NiC2O4���ȷֽ�Ļ�ѧ����ʽΪ___________________________________��
��4�������������֤��������ȫ______________________________________________��
����NiC2O4����ʱ���ϴ�ӳ���_________________________________________________��
��5����Al��NiO(OH)Ϊ�缫��NaOH��ҺΪ���Һ���һ�����͵�أ��ŵ�ʱNiO(OH)ת��ΪNi(OH)2����õ�ظ����ĵ缫��ӦʽΪ__________________________��
���𰸡������ܽ⣬����2Fe2++H2O2+2H+�T2Fe3++2H2OCO32-+2H+�TCO2��+2H2O ��CO32-+H2O![]() HCO3-+OH-��CO32-�����ᷴӦ��֮����ˮ��ʹ��Һ�ʼ��Դٽ�������ˮ�������ȫ2NiC2O4
HCO3-+OH-��CO32-�����ᷴӦ��֮����ˮ��ʹ��Һ�ʼ��Դٽ�������ˮ�������ȫ2NiC2O4 ![]() Ni2O3+3CO��+ CO2������ȡ�ϲ���Һ�������Թ��У��ټ�������������Һ�����Թ�����������˵���Ѿ�������ȫ�ز�������©���м�����ˮ��Һ���û��������ˮ��Ȼ���º��ظ�����2-3��Al+4OH--3e- = AlO2-+2H2O
Ni2O3+3CO��+ CO2������ȡ�ϲ���Һ�������Թ��У��ټ�������������Һ�����Թ�����������˵���Ѿ�������ȫ�ز�������©���м�����ˮ��Һ���û��������ˮ��Ȼ���º��ظ�����2-3��Al+4OH--3e- = AlO2-+2H2O
��������
��1��������ͼ���������ϣ����������ơ�þ�Ͻ�Ϊ������Ҫʹ֮������ӣ�Ӧ�ü����ܽ⣬�ܽ�������ٳ�ȥ��������ˣ��ʴ�Ϊ�������ܽ⣬���ˣ���2���ټ�˫��ˮ��Ŀ������������Fe3+����Ӧ�����ӷ���ʽΪ��2Fe2++H2O2+2H+�T2Fe3++2H2O���ڼ���̼������Һ�������ǵ���pH���ٽ�������ˮ�������ȫ���ʴ�Ϊ��CO32-+2H+�TCO2��+2H2O ��CO32-+H2O![]() HCO3-+OH-��CO32-�����ᷴӦ��֮����ˮ��ʹ��Һ�ʼ��Դٽ�������ˮ�������ȫ����3����������NiC2O42H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2���ʴ�Ϊ2NiC2O4
HCO3-+OH-��CO32-�����ᷴӦ��֮����ˮ��ʹ��Һ�ʼ��Դٽ�������ˮ�������ȫ����3����������NiC2O42H2O�����ȿ����и�����ˮ������NiC2O4��NiC2O4�ٷ���������ԭ��Ӧ��Ni��+2�����ߵ�+3�ۣ���C��+3�۽��͵�+2�ۣ�����Ҫ�����ɻ�����壬����һ����ΪCO2������Ni2O3��CO��CO2���ʴ�Ϊ2NiC2O4 ![]() Ni2O3+3CO��+ CO2������4����Ni2+������ȫ�����ټ��������Һʱ���ٳ��ֳ������ʴ�Ϊ������ȡ�ϲ���Һ�������Թ��У��ټ�������������Һ�����Թ�����������˵���Ѿ�������ȫ��ϴ�ӳ����IJ���Ϊ���ز�������©���м�����ˮ��Һ���û��������ˮ��Ȼ���º��ظ�����2-3�Σ���5���ڸ�ԭ�����AlΪ�������ŵ�����Al3+����NaOH��Һ������ת��ΪAlO2-���ʵ缫��ӦʽΪ��Al+4OH--3e- = AlO2-+2H2O��
Ni2O3+3CO��+ CO2������4����Ni2+������ȫ�����ټ��������Һʱ���ٳ��ֳ������ʴ�Ϊ������ȡ�ϲ���Һ�������Թ��У��ټ�������������Һ�����Թ�����������˵���Ѿ�������ȫ��ϴ�ӳ����IJ���Ϊ���ز�������©���м�����ˮ��Һ���û��������ˮ��Ȼ���º��ظ�����2-3�Σ���5���ڸ�ԭ�����AlΪ�������ŵ�����Al3+����NaOH��Һ������ת��ΪAlO2-���ʵ缫��ӦʽΪ��Al+4OH--3e- = AlO2-+2H2O��

����Ŀ���й���ѧԺ�ٷ�����2017��3�·���������ר�⡷�����������ϸ������ij����ַ�������ͼ��ʾ��

(1)������ͼ��Ϣ���Կ�������������������Ⱦ�������ɻ�������ʻ��ɵ�_________,
a��SO2 b��NOx c��VOCs d�� NH3
(2)������������ʻ����ȾԴ֮һ����������������ϡȼ����ϵͳ��Ҫ����ԭ����ͼ��ʾ��д��ϡȼ������NO��������Ҫ��Ӧ�ķ���ʽ_______��

(3)SO2��������Ⱦ�����Ҫ�ɷ�֮һ����Ϊһ�ֻ�ѧ���ʻ��õ����ʣ����ܷ������ַ�Ӧ���밴���±���ʾ���Ӳ�ͬ�Ƕ�����SO2��ͬ��Ļ�ѧ���ʣ�����ѧ����ʽ�����±��С�
SO2�������� | ��ѧ����ʽ | |
������� | ���������� | ��SO2 +H2O |
��SO2 + CaO = CaSO3 | ||
��_______________________ | ||
SԪ�� ��̬ | SΪ+4�� | ��_______________________ |
�� SO2 + 2H2S = 3S + 2H2O | ||
(4)��ѧ�Ҿ����о������й��������ԣ�����Ҫԭ����ͼ��ʾ��

���ж�A�Ļ�ѧʽ��˵���ж����ɣ�______________________��
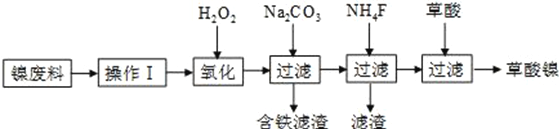
����Ŀ��ij���������������������� Fe2O3�� Fe3O4�� Al2O3�� CaO�� SiO2 �ȣ�Ϊԭ����ȡ������ Fe2O3��Ҫ��>99.2%�� CaO ����<0.01%�����乤����������(�������Լ����Թ���)��

��֪��������������� pH ���±���ʾ
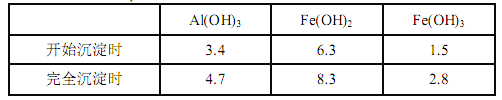
��1������ A ����Ҫ�ɷ���__________��
��2���ڹ��̢��пɹ۲쵽�����������ݣ���Һ��ɫ������dz���ܽ���ʵ����������ӷ���ʽ��__________��
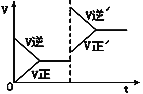
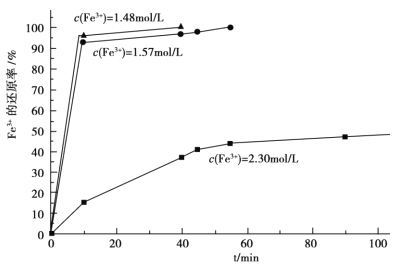
��3���ڹ��̢��У����������Һ A ϡ�Ͳ�ͬ������,����������Ĺ�������,�ó� Fe3+Ũ�ȡ���ԭ�ʺͷ�Ӧʱ��Ĺ�ϵ��ͼ��ʾ���������ʵ����˵����������ѡ��ϡ�ͺ�c(Fe3+)Ϊ 1.60mol/L ���ҵ�������______��
��4���ڹ��̢��У�����������ͬ�����£���ѡ���˲�ͬ���Ƽ�����ʵ�飬ʵ�����ݼ��±�������֪����Һ B �иƵĺ����� CaO ��Ϊ 290��310mg/L��
���Ƽ� | Na2SO3 | H2C2O4 | (NH4)2CO3 | Na2CO3 | NH4F |
����/g | 2 | 2 | 2 | 5 | 2 |
ʣ��CaO/mg/L) | 290 | 297 | 290 | 190 | 42 |
����ʵ������ ѡ�����˵ij��Ƽ����õ����� C ����Ҫ�ɷ���__________��
��5���ڹ��̢��У���Ӧ�¶���Ҫ������ 35�����£����˹��ߣ�����ܵ�ԭ����__________��
��6���ڹ��̢��У���Ӧ�Ļ�ѧ����ʽ��__________��