��Ŀ����
11������ʵ�������Ԥ��ʵ��Ŀ�Ļ�����ʵ����۶���ȷ���ǣ�������| ��� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��ij�л����м�������Cu��OH��2����Һ�����ȣ���ש��ɫ���� | ���л���һ����ȩ |
| B | ��CH3CH2Br��NaOH��Һ��ϼ��ȣ��ٵμ�AgNO3��Һ��δ����dz��ɫ���� | ֤��CH3CH2Brδ����ˮ�� |
| C | �ֱ���Ҵ��ͱ�����Һ�м�����ɫʯ����Һ���۲�����ɫ�ı仯 | �Ƚ��Ҵ��ͱ��ӵ�����ǿ�� |
| D | ��pH��ֽ�ⶨCH3COONa��Һ��pH | ֤��CH3COOH��������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A���ܺ�����������ͭ����Һ��ϼ�������ש��ɫ�����������к���ȩ����������ȩ����������ij����
B����ʵ����Ҫ�ȼ�ϡ�����кͼ
C���������Խ���������ʹ��ɫʯ����ɫ��
D�������������ǿ��ǿ���Σ�����Һ�����ԣ������ǿ�������Σ�����Һ�ʼ��ԣ�
��� �⣺A���ܺ�����������ͭ����Һ��ϼ�������ש��ɫ�����������к���ȩ����������ȩ����������ij�������Ը�ʵ����۴���A����
B����ʵ����Ҫ�ȼ�ϡ�����кͼʵ��ʱû�м�ϡ�����кͼ����ʵ�鲻�ɹ�������۴���B����
C���������Խ���������ʹ��ɫʯ����ɫ�����Բ�����ʯ����Һ����������ǿ������C����
D�������������ǿ��ǿ���Σ�����Һ�����ԣ������ǿ�������Σ�����Һ�ʼ��ԣ����Կ�����pH��ֽ�ⶨCH3COONa��Һ��pH�жϴ�����������ʣ���D��ȷ��
��ѡD��
���� ���⿼�黯ѧʵ�鷽�����ۣ�Ϊ��Ƶ���㣬�漰ȩ����±ԭ�Ӽ��鼰ǿ������ʵ��жϵ�֪ʶ�㣬��ȷʵ��ԭ�������������ǽⱾ��ؼ����״�ѡ����AC����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
6��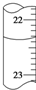 ����˵����ȷ���ǣ�������
����˵����ȷ���ǣ�������
��ʵ�������þƾ��Ƽ��ȵ���������Բ����ƿ���ձ�
��������100mlʯ�Ϳɽ�������250ml������ƿ��
�����Ƶ���ˮ��������ɫ���ƿ�У�������������
�ܲ�����Һ��CuSO4��ʯ��ˮ��һ��������ϣ�ʢ��������������
�����ζ����е�Һ����ͼ��ʾ���������ӦΪ23.65mL��
 ����˵����ȷ���ǣ�������
����˵����ȷ���ǣ���������ʵ�������þƾ��Ƽ��ȵ���������Բ����ƿ���ձ�
��������100mlʯ�Ϳɽ�������250ml������ƿ��
�����Ƶ���ˮ��������ɫ���ƿ�У�������������
�ܲ�����Һ��CuSO4��ʯ��ˮ��һ��������ϣ�ʢ��������������
�����ζ����е�Һ����ͼ��ʾ���������ӦΪ23.65mL��
| A�� | �٢� | B�� | �٢ۢ� | C�� | �ۢܢ� | D�� | �ڢۢ� |
16�����з�Ӧ�У���Ӧ�������������������ǣ�������
| A�� | ����ͨ�����ȵ�CuO��ĩ | B�� | ������̼ͨ��Na2O2��ĩ | ||
| C�� | ����Fe2O3�������ȷ�Ӧ | D�� | ��п��Ͷ��Cu��NO3��2��Һ |
3�������£����б�����ȷ���ǣ�������
| A�� | pH=2��CH3COOH��Һ��Ũ��С��pH=2�������Ũ�� | |
| B�� | ��NaHC03��Һ�м�������NaOH���壬������HCO3-��ˮ�⣬ʹc��HCO-���� �� | |
| C�� | Na2C03��Һ�У���c��Na+����C��CO32-����C��OH-��=C��HCO3-����C��H+�� | |
| D�� | ����������CH3COOH��Һ��NaOH��Һ��ϣ�������Һ�����ڣ�C��Na+��+c��H+��=C��CH3COO-��+C��OH-�� |
20��2011��3��11�����ձ������Ĵ�����У������˵�վ�����˺�й©�����ܱ�����Ŀ�����Ư���ŷ��������ʣ��Ի�������˼����Ӱ�죮���к��е��ͬλ��${\;}_{53}^{131}$I��${\;}_{53}^{131}$I�е��������ǣ�������
| A�� | 53 | B�� | 78 | C�� | 131 | D�� | 184 |
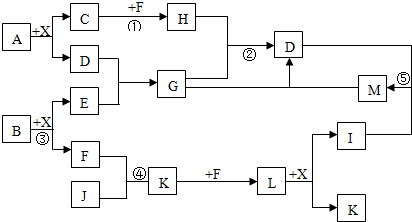
1��X��Y��Z��W����������һ�������¾�����ͼ��ʾ��ת����ϵ�������ж���ȷ���ǣ�������

| A�� | ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NaAlO2 | |
| B�� | ��ͼ�з�Ӧ��Ϊ��������ԭ��Ӧ����WΪһԪǿ��ʱ����X������NH3 | |
| C�� | ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ�ǽ�������ʱ����Z������CO2 | |
| D�� | ��ͼ�з�Ӧ��Ϊ������ԭ��Ӧ����WΪ��������ʱ����Z������FeCl3 |
 ��
�� $\stackrel{H_{2}O}{��}$2RCOOH
$\stackrel{H_{2}O}{��}$2RCOOH
 ���ݵķ�Ӧ�����Ǽӳɷ�Ӧ��G��Br2��CCl4��Һ��Ӧ�����п��ܵIJ��ﹲ��3�֣�
���ݵķ�Ӧ�����Ǽӳɷ�Ӧ��G��Br2��CCl4��Һ��Ӧ�����п��ܵIJ��ﹲ��3�֣� ��
�� ��
�� ��
��

 ��
��