��Ŀ����
����Ŀ����1��ʵ����5g�״�����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5 kJ����������д���״�ȼ�յ��Ȼ�ѧ����ʽ��___________________________��
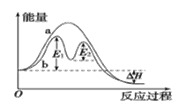
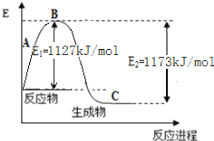
��2���ּ�֪N2(g)��H2(g)��Ӧ����1 molNH3(g)�����������仯ʾ����ͼ������������֪�������ݼ���N-H������Ϊ____________________kJ/mol ��
��ѧ�� | H-H |
|
����(kJ/mol) | 436 | 946 |
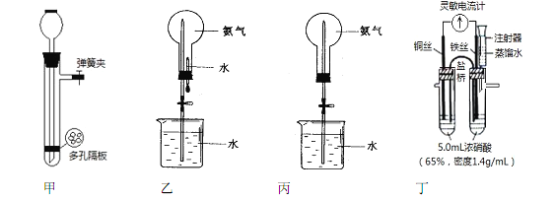
��3������ͼ��ʾװ�ý����к��Ȳⶨʵ�飬��ش��������⣺

����A������Ϊ ________________________ ��
ȡ30mLH2SO4��0.5mol��L-1����Һ��50mLNaOH��0.5mol��L-1����Һ��С�ձ��н����кͷ�Ӧ������ʵ���¶�ƽ������4.1������֪�кͺ����ɵ���Һ�ı�����Ϊ![]() ����Һ���ܶȾ�Ϊ1g/cm3��ͨ������ɵ��к��� ______ ��������С�����һλ��
����Һ���ܶȾ�Ϊ1g/cm3��ͨ������ɵ��к��� ______ ��������С�����һλ��
����ʵ����ֵ�����57.3kJ��mol-1��ƫ�������ƫ���ԭ������ǣ�����ĸ��______��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c��һ����NaOH��Һ����ʢ�������С�ձ���
ʵ����������60mLH2SO4��0.25mol��L-1����Һ��50mLNaOH��0.55 mol��L-1����Һ���з�Ӧ��������ʵ����ȣ����ų������� ______ �������������������������������к��� _____ ������������������������������50mL0.5mol��L-1�������H2SO4��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ�� ______ ������ƫ��������ƫС����������Ӱ��������
���𰸡�2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l) ![]() =-1452.8kJ/mol 391 ���β�������� -54.8kJ��mol-1 ab ����� ��� ƫС
=-1452.8kJ/mol 391 ���β�������� -54.8kJ��mol-1 ab ����� ��� ƫС
��������
��1��5g�״�����������ȫȼ�գ����ɶ�����̼�����Һ̬ˮʱ�ͷų�113.5 kJ����������2mol�״�ȼ�շų�����Ϊ![]() kJ=1452.8kJ���ʼ״�ȼ�յ��Ȼ�ѧ����ʽΪ��2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l)
kJ=1452.8kJ���ʼ״�ȼ�յ��Ȼ�ѧ����ʽΪ��2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l) ![]() =-1452.8kJ/mol��
=-1452.8kJ/mol��
��2����ͼ��֪��N2(g)��H2(g)��Ӧ����1 molNH3(g)���ų�������Ϊ��1173-1127��kJ=46kJ
����N-H������ΪakJ/mol����3a-��![]() ��946+
��946+![]() ��436��=46�����a=391��
��436��=46�����a=391��
��3������AΪ���β����������ȡ30mLH2SO4��0.5mol��L-1����Һ��50mLNaOH��0.5mol��L-1����Һ��С�ձ��н����кͷ�Ӧ������ˮ�����ʵ���Ϊ0.025mol����Һ������Ϊ80g������ʵ���¶�ƽ������4.1�����ų�������Q=4.18J/(g����)��80g��4.1��=1371J=1.371kJ������������1molˮ�ų�������Ϊ![]() =54.8kJ�����к��ȡ�H=-54.8kJ��mol-1��ʵ����ֵ�����57.3kJ��mol-1ƫ�ͣ�
=54.8kJ�����к��ȡ�H=-54.8kJ��mol-1��ʵ����ֵ�����57.3kJ��mol-1ƫ�ͣ�
a��ʵ��װ�ñ��¡�����Ч�������ʵ������в���������ʧ����Һ�¶�ƫ�ͣ��������������ƫ�ͣ��������⣻
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ��ᵼ���в������ʷ�Ӧ�������������ʼ�¶�ƫ�ߣ�����ʵ��ǰ���¶Ȳ�ƫ�ͣ��������������ƫ�ͣ��������⣻
c����ʵ���������ȷ����Ϊһ���Ի�ϣ��ʸò�����ʵ����Ӱ�죬���������⣻
�ʴ�Ϊab��
��Ӧ�ų����������������Լ�������Ķ����йأ�����60mLH2SO4��0.25mol��L-1����Һ��50mLNaOH��0.55 mol��L-1����Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��Ⱦ���ǿ���ǿ�Ӧ����1molˮʱ�ų����������к�����ȣ��ִ��������ᣬ����������ȣ�������50mL0.50molL1�������H2SO4��Һ��������ʵ�飬��÷�Ӧǰ���¶ȵı仯ֵ���С��
�ʴ�Ϊ������ȣ���ȣ�ƫС��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�