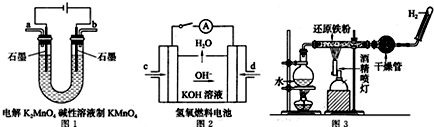
��Ŀ����
1������ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ��������| A�� | ��NH4HCO3��Һ�мӹ���NaOH��Һ�����ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| B�� | Fe3+��Һ�еμӹ����İ�ˮ��Fe3++3OH-=Fe��OH��3�� | |
| C�� | ����������ʵ���Ũ�ȵ�NaHCO3��Ba��OH��2��Һ��ϣ�HCO3-+Ba2++OH-�TBaCO3��+H20 | |
| D�� | NaHCO3��ˮ�⣺HCO3-+H2O=CO32-+H3O+ |
���� A�����߷�Ӧ���ɰ�����̼���ƺ�ˮ��
B��һˮ�ϰ���������ʣ�Ҫд��ѧʽ��
C������������ʵ���Ũ�ȵ�NaHCO3��Ba��OH��2��Һ��ϣ����߷�Ӧ����̼�ᱵ��NaOH��ˮ��
D��̼������ˮ������̼���NaOH����ˮ��̶Ƚ�С��
��� �⣺A�����߷�Ӧ���ɰ�����̼���ƺ�ˮ�����ӷ���ʽΪNH4++2OH-+HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CO32-+NH3��+2H2O����A����
B��һˮ�ϰ���������ʣ�Ҫд��ѧʽ�����ӷ���ʽΪFe3++3NH3��H2O�TFe��OH��3��+3NH4+����B����
C������������ʵ���Ũ�ȵ�NaHCO3��Ba��OH��2��Һ��ϣ����߷�Ӧ����̼�ᱵ��NaOH��ˮ�����ӷ���ʽΪHCO3-+Ba2++OH-�TBaCO3��+H20����C��ȷ��
D��̼������ˮ������̼���NaOH����ˮ��̶Ƚ�С��ˮ�ⷽ��ʽΪHCO3-+H2O?H2CO3+OH-����D����
��ѡC��
���� ���⿼�����ӷ���ʽ�����жϣ�Ϊ��Ƶ���㣬��ȷ���ʵ����ʼ����ӷ���ʽ��д���ɽ�𣬳���������ˮ�⡢������ԭ��Ӧ���Ͽ��飬�״�ѡ����A��

��ϰ��ϵ�д�
�����Ŀ
6�����л������У�������������ǣ�������
| A�� | Na2SO4 | B�� | NaOH | C�� | HNO3 | D�� | FeO |
10�����ӱ����õ�ij��Ŧ�۵�صĵ缫����ΪZn��Ag2O���������Һ��KOH��Һ������ܷ�ӦʽΪ��Zn+Ag2O�TZnO+2Ag������˵��������ǣ�������
| A�� | �õ�ص�������Ag2O��������Zn | |
| B�� | �õ�ظ����ĵ缫��ӦʽΪ��Zn+2OH--2e-�TZnO+H2O | |
| C�� | �����ϸõ�ع���һ��ʱ�����Һ��KOH��Ũ�Ȳ��� | |
| D�� | �õ�ع���ʱ��������е��������������ƶ� |
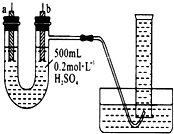 �����ṩ��п����ͭƬ��500 mL 0.2 mol/L��H2SO4��Һ�����ߡ�1 000 mL��Ͳ��������ͼװ�����ⶨп��ϡ���ᷴӦʱ����ij��ʱ����ͨ�����ߵĵ��ӵ����ʵ�����
�����ṩ��п����ͭƬ��500 mL 0.2 mol/L��H2SO4��Һ�����ߡ�1 000 mL��Ͳ��������ͼװ�����ⶨп��ϡ���ᷴӦʱ����ij��ʱ����ͨ�����ߵĵ��ӵ����ʵ�����