ЬтФПФкШн
ЁОЬтФПЁППзШИЪЏЕФжївЊГЩЗжЮЊCu2(OH)2CO3ЃЌЛЙКЌЩйСПFeЁЂSiЕФЛЏКЯЮяЁЃЪЕбщЪввдПзШИЪЏЮЊдСЯжЦБИCuCl2ЁЄ3H2OМАCaCO3ЕФВНжшШчЯТЃК
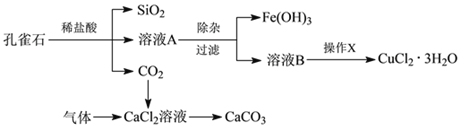
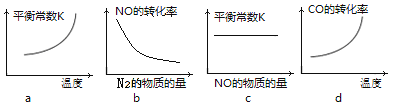
ЮЊНтОігаЙиЮЪЬтЃЌаЫШЄаЁзщЭЌбЇВщЕУгаЙиЮяжЪГСЕэЕФpHЪ§ОнШчЯТЃК
ЮяжЪ | pH (ПЊЪМГСЕэ) | pH(ЭъШЋГСЕэ) |
Fe(OH)3 | 1.9 | 3.2 |
Fe(OH)2 | 7.0 | 9.0 |
Cu(OH)2 | 4.7 | 6.7 |
ЃЈ1ЃЉдкГ§дгЙ§ГЬЪБЮЊСЫГ§ШЅFe2+ЃЌГЃМгШыТЬЩЋбѕЛЏМСЃЌЪЙFe2+бѕЛЏЮЊFe3+ЃЌДЫЙ§ГЬЩцМАЕФРызгЗНГЬЪНЮЊ________ЁЃ
ЃЈ2ЃЉШЛКѓдйМгШыЪЪЕБЮяжЪЕїНкШмвКЕФpHжС_________ (ЬюаДЗЖЮЇ)ЃЌЪЙFe3+зЊЛЏЮЊFe(OH)3ЃЌПЩвдДяЕНГ§ШЅFe3+ЃЌЕїећШмвКpHВЛПЩбЁгУЯТСажаЕФ________ЁЃ
A.NaOH B.NH3ЁЄH2O C.CuO D.Cu(OH)2
ЃЈ3ЃЉМьбщFe(OH)3ЪЧЗёГСЕэЭъШЋЕФЪЕбщЗНАИЪЧ________________________ЁЃ
ЃЈ4ЃЉВйзїXАќРЈ________ЁЂ________ЁЂЙ§ТЫКЭЯДЕгЕШЁЃCuCl2ЁЄ3H2OМгШШзЦЩеЕФзюжеВњЮяЕФЛЏбЇЪНЪЧ ______________ЁЃ
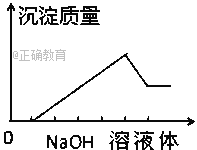
ЃЈ5ЃЉФГжжЮИвЉЦЌЕФжЮЫсМСЮЊCaCO3ЃЌИУвЉЦЌжаCaCO3жЪСПЗжЪ§ЕФВтЖЈВНжшШчЯТЃК
aЃЎХфжЦ0.1molЁЄLЃ1ЕФHClШмвККЭ0.1molЁЄLЃ1ЕФNaOHШмвКИї250mLЁЃ
bЃЎШЁ0.6gФЅЫщКѓЕФЮИвЉгкзЖаЮЦПжаЁЃ
cЃЎЯђзЖаЮЦПФкМгШы25.00mL 0.1 molЁЄLЃ1ЕФHClШмвКЁЃ
dЃЎвдЗгЬЊЮЊжИЪОМСЃЌгУ0.1molЁЄLЃ1ЕФNaOHШмвКЕЮЖЈЃЌжСДяЕНЕЮЖЈжеЕуЁЃ
eЃЎжиИДbcdШ§ВНЕФВйзї2ДЮЁЃ
ЂйЖСЪ§ЪБЃЌШєЕЮЖЈЧАЦНЪгЃЌЕЮЖЈКѓбіЪгЃЌдђЫљВтCaCO3ЕФжЪСПЗжЪ§НЋ________(бЁЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАЮогАЯьЁБ)ЁЃ
ЂкШєЕЮЖЈжаNaOHШмвКЕФЦНОљгУСПЮЊ15.00mLЃЌдђЮИвЉжаЬМЫсИЦЕФжЪСПЗжЪ§ЮЊ_______ЁЃ
ЁОД№АИЁП H2O2+2Fe2++2H+ =2Fe3++2H2O 3.2Ѓ4.7 AB ШЁЩйСПЩЯВуЧхвКЃЌЕЮМгKSCNШмвКЃЌШєВЛГіЯжбЊКьЩЋЃЌБэУїFe(OH)3ГСЕэЭъШЋ еєЗЂХЈЫѕ РфШДНсОЇ CuO ЦЋаЁ 8.3ЃЅ
ЁОНтЮіЁПЃЈ1ЃЉТЬЩЋбѕЛЏМСЪЧЙ§бѕЛЏЧтЃЌЫљвдЦфбѕЛЏбЧЬњРызгЕФЗНГЬЪНЮЊH2O2+2Fe2++2H+ =2Fe3++2H2OЁЃ
ЃЈ2ЃЉЕїНкpHгІИУНЋЬњРызгЖМзЊЛЏЮЊЧтбѕЛЏЬњГСЕэЃЌЫљвдгІИУДѓгк3.2ЃЌЕЋЪЧЭЌЪБВЛФмГСЕэЧтбѕЛЏЭЃЌЫљвдгІИУаЁгк4.7ЁЃЪЙгУЧтбѕЛЏФЦЛђАБЫЎЕїНкpHЃЌЖМКмШнвзЕїНкЙ§СПЪЙШмвКжаЕФЭРызгзЊЛЏЮЊЧтбѕЛЏЭГСЕэЁЃЫљвдABВЛПЩбЁЁЃЖјCuOКЭCu(OH)2ЖМВЛШмгкЫЎЃЌжБНгМгЙ§СПвВВЛЛсЪЙЭРызгГСЕэЃЌЫљвдCDЖМЪЧПЩвдбЁдёЕФЁЃ
ЃЈ3ЃЉМьбщFe(OH)3ЪЧЗёГСЕэЭъШЋЕФЪЕбщЗНАИЪЧОЭЪЧМьбщШмвКжаЪЧЗёЛЙгаЬњРызгЃЌЫљвдЮЊЃКЩйСПЩЯВуЧхвКЃЌЕЮМгKSCNШмвКЃЌШєВЛГіЯжбЊКьЩЋЃЌБэУїFe(OH)3ГСЕэЭъШЋЁЃ
ЃЈ4ЃЉЮЊСЫБЃжЄФмЕУЕНДјгаНсОЇЫЎЕФбЮЃЌЭЌЪБОЁСПМѕаЁТШЛЏЭЕФЫЎНтЃЌЫЎШмвКгІИУОЃКеєЗЂХЈЫѕЁЂРфШДНсОЇЁЂЙ§ТЫЯДЕгЃЌИЩдяЕУЕНОЇЬхЃЈВЛбЁдёеєИЩЕФжївЊЮЪЬтЪЧЃЌеєИЩЙ§ГЬжаЭРызгПЩФмЫЎНтЃЌЖјЧвПЩФмЪЇШЅНсОЇЫЎЃЉЁЃCuCl2ЁЄ3H2OМгШШзЦЩеЪБЃЌПМТЧЭРызгЕФЫЎНтЃЌЦфзюжеВњЮяЮЊCuOЁЃ
ЃЈ5ЃЉЂйЖСЪ§ЪБЃЌШєЕЮЖЈЧАЦНЪгЃЌЕЮЖЈКѓбіЪгЃЌдђЖСЪ§ЛсЦЋДѓЃЌМДНЋгыбЮЫсЗДгІЕФЧтбѕЛЏФЦЫуЖрСЫЃЌвВОЭНЋгыЬМЫсИЦЗДгІКѓЪЃгрЕФбЮЫсЫуЖрСЫЁЃвђЮЊМгШыЕФбЮЫсзмСПЪЧЖЈжЕЃЌЫљвдНЋгыЬМЫсИЦЗДгІЕФбЮЫсЫуЩйСЫЃЌвВОЭНЋЬМЫсИЦЫуЩйСЫЃЌЫљВтCaCO3ЕФжЪСПЗжЪ§НЋЦЋаЁЁЃ
ЂкЕЮЖЈжаNaOHШмвКЕФЦНОљгУСПЮЊ15.00mLЃЌМДЧтбѕЛЏФЦЮЊ0.015ЁС0.1=0.0015molЃЌЫљвджаКЭЕФбЮЫсЮЊ0.0015molЃЌЫљвдгыЬМЫсИЦЗДгІЕФбЮЫсЮЊЃК0.025ЁС0.1Ѓ0.0015=0.001molЃЌЫљвдЬМЫсИЦЮЊ0.0005molЃЌжЪСПЮЊ0.05gЃЌжЪСПЗжЪ§ЮЊ0.05ЁТ0.6=8.3%ЁЃ

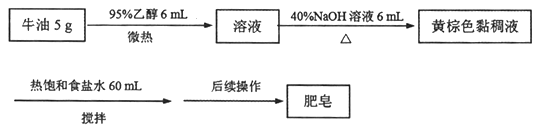
 жЧШЄКЎМйзївЕдЦФЯПЦММГіАцЩчЯЕСаД№АИ
жЧШЄКЎМйзївЕдЦФЯПЦММГіАцЩчЯЕСаД№АИЁОЬтФПЁПЭъГЩЯТСаЪЕбщЫљбЁдёЕФзАжУЛђвЧЦї(МаГжзАжУвбТдШЅ)е§ШЗЕФЪЧ
бЁЯю | A | B | C | D |
ЪЕбщ | гУCCl4ЬсШЁфхЫЎжаЕФBr2 | ДгЪГбЮЫЎжаЬсШЁNaClЙЬЬх | ДгKIКЭI2ЕФЙЬЬхЛьКЯЮяжаЛиЪеI2 | ХфжЦ100 mL 0.100 0 molЁЄL-1 K2Cr2O7ШмвК |
зАжУЛђвЧЦї |
|
|
|
|
A. A B. B C. C D. D
ЁОЬтФПЁПШэУЬПѓЕФжївЊГЩЗжЪЧMnO2ЃЌЛЙКЌгаЩйСПН№ЪєЬњЁЂУОЁЂТСЁЂаПЁЂЭЕФЛЏКЯЮяЕШдгжЪЁЃЛЦЬњПѓЕФжївЊГЩЗжЪЧFeS2ЃЌЛЙКЌгаЙшЁЂТСЕФбѕЛЏЮяЕШдгжЪЁЃЙЄвЕЩЯгУШэУЬПѓжЦБИЬМЫсУЬВЂЛиЪеСђЫсяЇЃЌЦфжївЊСїГЬШчЯТЃК

вбжЊН№ЪєРызгДгПЊЪМаЮГЩЧтбѕЛЏЮяГСЕэЃЌЕНГСЕэЪБШмвКЕФpHШчЯТБэЃК
Н№ЪєРызг | Fe2+ | Fe3+ | Al3+ | Cu2+ | Mn2+ |
ПЊЪМГСЕэpH | 7.5 | 2.7 | 4.1 | 5.9 | 8.8 |
ЭъШЋГСЕэpH | 9.5 | 3.7 | 5.4 | 6.9 | 10.8 |
ЃЈ1ЃЉЬсИпНўГіТЪЕФПЩВЩШЁЕФДыЪЉга____ЁЃ
a.ЪЪЕБЩ§ИпЮТЖШ b.НСАш c.МгЪЪСПДПМю d.МгбЙ
ЃЈ2ЃЉНўШЁЭъГЩКѓЃЌШЁНўШЁвКЩйаэЃЌМгШыKSCNШмвКЮоУїЯдЯжЯѓЃЌдђНўШЁЪБЗЂЩњЕФжївЊЗДгІЕФЛЏбЇЗНГЬЪНЪЧ__________________________ЁЃ
ЃЈ3ЃЉЕїНкpHЮЊ5.4ЁЋ5.8ЕФФПЕФЪЧ______________ЁЃ
ЃЈ4ЃЉТЫдќ3ЕФжївЊГЩЗжЕФЛЏбЇЪНЪЧ_______ЁЃ
ЃЈ5ЃЉВЩгУ50ЁцЬМЛЏЕФдвђЪЧ_________________________________ЁЃИУЙ§ГЬЗЂЩњЗДгІЕФРызгЗНГЬЪНЪЧ__________________ЁЃ
ЃЈ6ЃЉМьбщЬМЫсУЬВњЦЗЭъШЋЯДОЛЕФЗНЗЈЪЧ_______________________________ЁЃ