��Ŀ����
15�����з�Ӧ�����ӷ���ʽ��ȷ���ǣ�������| A�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+=SO2��+H2O | |
| B�� | ��Ư����Һ�м������Ũ���ClO-+H+=HClO | |
| C�� | �����ܻ�ԭ����NaBH4��ˮ��Ӧ�õ�NaBO2��BH4-+2H2O=BO2-+4H2�� | |
| D�� | ��FeSO4��Һ�м���Na2O2��2Na2O2+2Fe2++2H2O=4Na++2Fe��OH��2��+O2�� |
���� A��ϡ������������ԣ��ܽ����������������Ϊ��������ӣ�
B��������ƿ��Ժ�����֮�䷴Ӧ����������
C��NaBH4����ˮ����ˮ��Ӧ��NaBH4+2H2O=NaBO2+4H2����
D��Na2O2Ͷ��FeSO4��Һ����������������ˮ��Ӧ�����������ƺ�����������������FeSO4��Һ�������ֽⷴӦ����������������������������������������������������
��� �⣺A����ϡHNO3�еμ�Na2SO3��Һ��ϡ������������ԣ��ܽ����������������Ϊ��������ӣ�������������������壬��A����
B����Ư����Һ�м������Ũ���ᣬ����������Ӻ������������Ի����»ᷢ����Ӧ�õ���������B����
C�����ܻ�ԭ����NaBH4��ˮ��Ӧ�õ�NaBO2��NaBH4+2H2O=NaBO2+4H2������BH4-+2H2O=BO2-+4H2������C��ȷ��
D����FeSO4��Һ�м���Na2O2������������ˮ��Ӧ�����������ƺ������������������Ȼ������������ֽⷴӦ����������������������������������������������������ʵ�����������ݲ������ų��������ȣ���Һ�����а�ɫ����������Ȼ����ɫѸ�ٱ�����ã��ɻ���ɫ��ɺ��ɫ�������漰�Ļ�ѧ����ʽ����Ϊ��2Na2O2+2H2O�T4NaOH+O2����FeCl2+2NaOH�TFe��OH��2��+2NaCl��4Fe��OH��2+O2+2H2O�T4Fe��OH��3��ѡ���������ܽ���������������������ͬʱ���ɣ���D����
��ѡC��
���� ���⿼�������ӷ���ʽ����д����Ŀ�ѶȲ�����Ϥ��Ӧ��ʵ���ǽ���ؼ���ע�����ӷ���ʽ��ѭ�����غ㡢����غ㶨�ɣ�

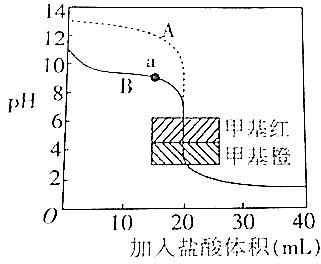
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �����£�pH=3�ļ�����Һ��c��H+����pH=11�İ�ˮ��Һ����ˮ���������c��OH-����� | |
| B�� | �к� 0.1 mol•L-1 �������к� 0.01 mol•L-1�Ĵ�������ͬ�ּ���Һ�����ʵ�����ͬ | |
| C�� | ��NH4Cl��Һ����������NaOH���壬��Һ�ĵ�����������ǿ | |
| D�� | ��ʯ��ˮ�м�������CaO���ָ������º���Һ��c��OH-��������������ǿ |
| A�� | Na2S��Һ��c��Na+����c��HS-����c��OH-����c ��H2S�� | |
| B�� | Na2C2O4��Һ��c ��OH-��=c��H+��+c��HC2O4-��+2c ��H2C2O4�� | |
| C�� | Na2CO3��Һ��c ��Na+��+c ��H+��=2c ��CO32-��+c ��OH-�� | |
| D�� | CH3COONa��CaCl2�����Һ��c ��Na+��+c ��Ca2+��=c ��CH3COO-��+c ��CH3COOH��+2c ��Cl-�� |
| A�� | �Ȼ�������ˮ�������ᣬ�����ǵ���ʣ��Ȼ����Ƿǵ���� | |
| B�� | NH3 ����ˮ����NH3•H2O��NH3•H2O�ǵ���ʣ�NH3�Ƿǵ���� | |
| C�� | ����ʵ�ˮ��Һһ���ܵ��磬�ǵ���ʵ�ˮ��Һһ�����ܵ��� | |
| D�� | Һ̬CH3COOH���ܵ��磬���Դ����Ƿǵ���� |
| A�� | 2NO��g��+2CO��g���TN2��g��+2CO2��g���ڳ��������Է����У���÷�Ӧ�ġ�H��0 | |
| B�� | �����ĸ�ʴ�����У����ⸯʴ��������ʴ���ܻ�ͬʱ���� | |
| C�� | N2��g��+3H2��g���T2NH3��g����H��0��������������ʱ�����¶ȣ���Ӧ����v ��H2����������ƽ��ת���ʾ����� | |
| D�� | �����£�pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�������ϣ���ҺpH��7 |
 ��������Ϊ������ȩ����
��������Ϊ������ȩ����
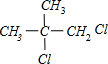 PMAA
PMAA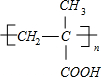
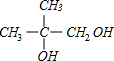 +O2$��_{��}^{Cu}$2
+O2$��_{��}^{Cu}$2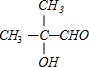 +2H2O��
+2H2O�� $��_{��}^{Ũ����}$CH2=C��CH3��-COOH+H2O��
$��_{��}^{Ũ����}$CH2=C��CH3��-COOH+H2O��