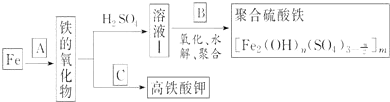
��Ŀ����
13���ྦྷ�裨�赥�ʵ�һ�֣�����Ϊ�����Ӵ��õĻ�ʯ�����Ʊ��и�������SiCl4Ϊ������������Ⱦ�ܴ�����ˮǿ��ˮ�����������H4SiO4���ų��������ȣ��о���Ա����SiCl4ˮ�����ɵ�����ͱ���ۣ���Ҫ�ɷ�ΪBaCO3���Һ�������þ�����ӣ����Ʊ�BaCl2•2H2O�������������£�
��֪���ٳ�����Fe3+��Mg2+��ȫ������PH�ֱ���3.4��12.4��
��BaCO3����Է���������197��BaCl2��2H2O����Է���������244���ش��������⣺
��1��SiCl4����ˮ�ⷴӦ�Ļ�ѧ����ʽSiCl4+4H2O=H4SiO4��+4HCl
��2��SiCl4��g����H2��ԭ����ȡ���ȺܸߵĹ裬����Ӧ����1mol����ת��ʱ����59kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪSiCl4��g��+2H2��g��=Si��s��+4HCl��g����H=+236 kJ/mol
��3���ӱ���۵���PH=7��������ʹBaCO3ת��ΪBaCl2��ʹFe3+ ��ȫ������
��4����������A�����ӷ���ʽMg2++2OH-=Mg��OH��2������
��5��BaCl2��Һ������Ũ�������½ᾧ�����ˡ�ϴ�ӣ��پ���ո����õ�BaCl2��2H2O��
��6��10�ֺ�78.8% BaCO3�ı�������������������BaCl2��2H2O9.76�֣�
���� ���̷�����֪���Ȼ�������¶�40��C������ˮˮ������ԭ������Ȼ��⣬���˵õ�������Һ�����뱵�����Ҫ�ɷ�ΪBaCO3���Һ�������þ�����ӣ�������ҺpH=7��ʹBaCO3ת��ΪBaCl2��ͬʱʹFe3+��ȫ���������˵õ�����Ϊ������������Һ��������������Һ������ҺpH=12.5������70��C���õ�������þ���������˵õ���ҺΪ�Ȼ�����Һ����Ũ������ȴ�ᾧ������ϴ�ӵõ��Ȼ������壻
��1���Ȼ���ˮ������ԭ������Ȼ��⣻
��2�������Ȼ�ѧ����ʽ��д�����õ���SiCl4�Ʊ�����Ȼ�ѧ����ʽ��ע���ʱ�ļ��㣻
��3��pH=3.4ʱ������������ת��Ϊ����������������PH=7ʱ�ܴ�ʹ���������ӳ�����ȫ��
��4����������������������þ��ȫ����������Һ��PHֵȷ������A�ijɷ֣�
��5������Һ����ȡ����ķ����ǣ�����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�
��6����ԭ���غ�֪��1molBaCO3������1molBaCl2•2H2O��n��BaCl2•2H2O��=n��BaCO3�����Դ˼��㣮
��� �⣺��1���Ȼ���ˮ������ԭ������Ȼ��⣬ˮ�ⷽ��ʽΪ��SiCl4+4H2O=H4SiO4��+4HCl����
�ʴ�Ϊ��SiCl4+4H2O=H4SiO4��+4HCl����
��2����H2��ԭSiCl4��������ȡ���ȺܸߵĹ裬����Ӧ����1mol����ת��ʱ����59KJ������ת��4mol������������236KJ����Ӧ���Ȼ�ѧ����ʽ��SiCl4��s��+2H2��g��=Si��s��+4HCl��g����H=+236kJ/mol��
�ʴ�Ϊ��SiCl4��s��+2H2��g��=Si��s��+4HCl��g����H=+236kJ/mol��
��3��pH=3.4ʱ��������������ȫ���ɳ�����ʹFe3+��ȫ�����������̼�ᱵ��Ӧ�����Ȼ����Ͷ�����̼��ˮ���ӱ���۲�����pH=7��������ʹBaCO3ת��ΪBaCl2��ͬʱʹʹFe3+��ȫ������
�ʴ�Ϊ��ʹFe3+��ȫ������
��4����pH=3.4ʱ����������ȫ���ɳ�������pH=12.4ʱ��þ������ȫ��������������þ��Mg��OH��2�����Ե�pH=12.5ʱ������A�ijɷ���������þ����Ӧ�����ӷ���ʽΪ��Mg2++2OH-=Mg��OH��2����
�ʴ�Ϊ��Mg2++2OH-=Mg��OH��2����
��5��BaCl2•2H2OΪ�ᾧˮ���Ϊ�˷�ֹ�ᾧˮ��ɢʧ��ͨ������Һ����ȡ����ķ����ǽ��½ᾧ����������Ũ�������½ᾧ�����ˡ�ϴ�ӣ�
�ʴ�Ϊ������Ũ�������½ᾧ��
��6����ԭ���غ�֪��1molBaCO3������1molBaCl2•2H2O��n��BaCl2•2H2O��=n��BaCO3��
m��BaCl2•2H2O��=n��BaCl2•2H2O����M��BaCl2•2H2O��=$\frac{10t��78%}{197g/mol}$��244g/mol=9.76t��
�ʴ�Ϊ��9.76��
���� ���⿼��BaCl2•2H2O�Ʊ�ʵ�飬���ؿ������ʷ�����ᴿ�ķ����ͻ���������Ӧ�ã���ȷ�������Һ����ȡ����ķ������Լ�����ԭ���غ�����������ļ���ȣ���Ŀ�Ѷ��еȣ�

| A�� | CH2=CH2+Br2��CCl4���� | B�� | CH2=CH-CH2-CH3+HCl$��_{��}^{����}$ | ||
| C�� | C��CH3��4+Cl2$\stackrel{����}{��}$ | D�� | n CH3-CH=CH2$��_{��}^{����}$ |
| A�� | ��ϡHNO3�еμ�Na2SO3��Һ��SO32-+2H+=SO2��+H2O | |
| B�� | ��Ư����Һ�м������Ũ���ClO-+H+=HClO | |
| C�� | �����ܻ�ԭ����NaBH4��ˮ��Ӧ�õ�NaBO2��BH4-+2H2O=BO2-+4H2�� | |
| D�� | ��FeSO4��Һ�м���Na2O2��2Na2O2+2Fe2++2H2O=4Na++2Fe��OH��2��+O2�� |

��֪��ijЩ�������������pH�����ʾ��
| �������� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Mg��OH��2 | 9.3 | 10.8 |
| Fe��OH��2 | 7.6 | 9.6 |
| Fe��OH��3 | 2.7 | 3.7 |
| Al��OH��3 | 3.7 | 4.7 |
��1�����������У����ӿ����ʱ�Ļ�ѧ��Ӧ���ʣ���������ֿ��еĴ�ʩ���ʵ����¡�����þ��������衢���ʵ���������Ũ�ȣ�
��2�������NaClO����Mn2+��Ӧ��Mn2++ClO-+H2O=MnO2��+2H++Cl-������һ������Ҳ�ᱻNaClO������������ˮ�⣬�÷�Ӧ�����ӷ���ʽΪ2Fe2++ClO-+5H2O=2Fe��OH��3��+Cl-+4H+��
��3����������Ҫ�ɷݳ�����Fe��OH��3��Al��OH��3��MnO2�⣬����SiO2��CaSO4��
��4����֪MgSO4��CaSO4���ܽ�������
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
��5��ʵ�����ṩ����þ�100g���õ���MgSO4•7H2OΪ172.2g������MgSO4•7H2O�IJ���Ϊ70%����������λ��Ч���֣�
��6������þ��������Ȼˮ���������ĵ绯ѧ���������ͼ2����ʾ��ͼ��������Ӧ��ע
 ��
�� ��
�� 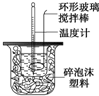 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺��1�����β����������������ʹ��Һ���»�Ͼ��ȣ���֤��Һ���µ��¶ȴﵽһ�£�
��2����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���кͣ����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���ƫС���ƫ��ƫС�����䡱����
��3����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼����ԭʼ���ݣ�?
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �²t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 30.6 | 5.5 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��4�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ƫ���ƫ��ƫС�����䡱������ԭ�����ô���������ᣬ�������Ҫ������������ɲ�õ��к�����ֵƫС�����к���Ϊ��ֵ�������к���ƫ��?
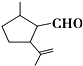 �л���A��һ����Ҫ�Ļ���ԭ�ϣ���ṹ��ʽ��ͼ�����м���A�й����ŵ��Լ���˳����ȷ���ǣ�������
�л���A��һ����Ҫ�Ļ���ԭ�ϣ���ṹ��ʽ��ͼ�����м���A�й����ŵ��Լ���˳����ȷ���ǣ�������| A�� | �ȼ�KMnO4������Һ���ټ�������Һ���� | |
| B�� | �ȼ���ˮ���ټ�KMnO4������Һ | |
| C�� | �ȼ�������Һ���ȣ��ټ���ˮ | |
| D�� | �ȼ�������������Cu��OH��2����Һ���ȣ��ữ���ټ���ˮ |
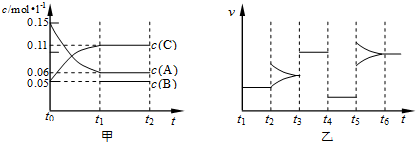
 2C��g��+B��g����H=+150akJ/mol��
2C��g��+B��g����H=+150akJ/mol��