��Ŀ����
5��������������õ���һ�ָ��ܵ��--��п���أ���缫�ֱ���Ag2O��Zn�����Һ��KOH��Һ���ŵ�ʱ���õ�صĵ缫��ӦʽΪZn+2OH--2e-=Zn��OH��2��Ag2O+H2O+2e-=2Ag+2OH-������˵������ȷ���ǣ���������пΪ������Ag2OΪ������
�ڷŵ�ʱ������������ҺOH-Ũ������
�۹���ʱ������Ag2O�������·����Zn����
����Һ�������������������ƶ����������������ƶ���
| A�� | ֻ�Т� | B�� | ֻ�Тڢ� | C�� | ֻ�Т٢ۢ� | D�� | ֻ�Т٢ڢ� |
���� ���ݵ缫��Ӧʽ֪��Znʧ���ӻ��ϼ�����Ϊ������Ag2O���������ŵ�ʱ���������������������ӡ��������������������ӣ��������Һ���������������ƶ������������ƶ����ݴ˷������
��� �⣺�ٵ缫��Ӧʽ��ZnԪ�ػ��ϼ���0�۱�Ϊ+2�ۡ�AgԪ�ػ��ϼ���+1�۱�Ϊ0�ۣ�����пΪ������Ag2OΪ����������ȷ��?
�ڷŵ�ʱ�������������������������ɣ�������ҺpHֵ����������ȷ��?
�۷ŵ�ʱ�������������������������Ag2O�������·����Zn��������ȷ��?
�ܷŵ�ʱ�����ӴӸ����ص�����������������Һ���������������ƶ��������������������ƶ����ʴ���
��ѡD��
���� ���⿼����ԭ���ԭ�������ݵ缫��Ӧʽ��Ԫ�ػ��ϼ۱仯ȷ�����������ٽ�������ƶ������������Ϸ����ķ�Ӧ���������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
15����֪ϩ����Ȳ���ڳ����������·������·�Ӧ��
CH3-CH�TCH-CH2-CH�TCH2��CH3CHO+OHC-CH2-CHO+HCHO
CH3-C��C-CH2-C��CH-��CH3COOH+HOOCCH2COOH+HCOOH ij������ʽΪC10H10���ڳ��������·�����Ӧ��C10H10��CH3COOH+3HOOC-CHO+CH3CHO��
�����ж���ȷ���ǣ�������
CH3-CH�TCH-CH2-CH�TCH2��CH3CHO+OHC-CH2-CHO+HCHO
CH3-C��C-CH2-C��CH-��CH3COOH+HOOCCH2COOH+HCOOH ij������ʽΪC10H10���ڳ��������·�����Ӧ��C10H10��CH3COOH+3HOOC-CHO+CH3CHO��
�����ж���ȷ���ǣ�������
| A�� | ��ṹ�к���4��̼̼˫����4��̼̼���� | |
| B�� | �����к���4��̼̼˫����2��̼̼���� | |
| C�� | �����ṹ��ʽ�ǣ�CH3-C��C-CH=CH-C��C-CH=CH-CH3 | |
| D�� | ��ṹ�к���2��̼̼˫����4��̼̼���� |
13���ձ����¹��ͷų��ļ����˹������Ժ���131I������������״�ٰ�����127Iȴ���������ģ�127I�������״�����շ�����131I�����κ�127IΪ 30mg•kg-1������һ���������Լ3kg����Ԥ��Ч���� �����й�˵������ȷ���ǣ�������
| A�� | 131I��127I��Ϊͬλ�� | |
| B�� | ��ͬ���ʵ�����131I��127I����������ͬ | |
| C�� | 131I��127I �����ֲ�ͬ���� | |
| D�� | ���õ��ζ�Ԥ�����������Ч���� |
20������������Һ����������������������Һ�� �ڱ��ͼױ��� ���廯�ƺ͵������ˮ��Һ���������ϸ����Һ����ȷ���������ǣ�������
| A�� | ��Һ����ȡ������ | B�� | ��ȡ������Һ | C�� | ��Һ��������ȡ | D�� | ������ȡ����Һ |
10��CH3OH��C2H5OH�Ļ������ŨH2SO4���ȣ��������ɵ��л�������У�������
| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
14�����ж��ڷ�Ӧ3NO2+H2O=2HNO3+NO��˵������ȷ���ǣ�������
| A�� | �������뻹ԭ����������Ϊ1��2 | |
| B�� | NO2��������ˮ�ǻ�ԭ�� | |
| C�� | ����1molNO����6mol���ӷ���ת�� | |
| D�� | �������뻹ԭ�������ʵ�����Ϊ2��1 |
15����1mol/L�ģ�NH4��2SO4��Һ�У������й�����Ũ�ȴ�С�Ƚ��У���ȷ���ǣ�������
| A�� | c��NH4+��=2c��SO42-����c��H+��=c��OH-�� | B�� | c��NH4+����c��SO42-����c��H+����c��OH-�� | ||
| C�� | c��SO42-����c��NH4+����c��H+����c��OH-�� | D�� | c��SO42-����c��NH4+����c��OH-����c��H+�� |
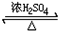 CH3COOC2H5+H2O��Ӧ����ȡ����Ӧ��
CH3COOC2H5+H2O��Ӧ����ȡ����Ӧ�� ��Ӧ���ͼӾ۷�Ӧ��
��Ӧ���ͼӾ۷�Ӧ��