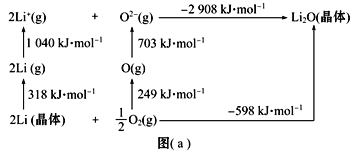
��Ŀ����
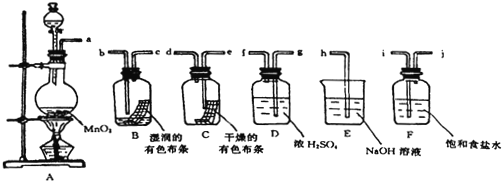
����Ŀ����֪NH4HCO3![]() NH3����CO2����H2O����Ϊ�˽����������ơ�̼����淋�NaCl�ᴿ�����Ƶô�����NaCl��Һ��ijѧ���������ʵ�鷽����
NH3����CO2����H2O����Ϊ�˽����������ơ�̼����淋�NaCl�ᴿ�����Ƶô�����NaCl��Һ��ijѧ���������ʵ�鷽����
��1��������������IJ���������_________________________________��
��2��������Ϊʲô�������ᱵ��Һ����������________________________________��
��3�����в����ں�����ж�SO![]() �ѳ�����������___________________________��
�ѳ�����������___________________________��
��4�������۵�Ŀ����__________������Ӧ�����ӷ���ʽΪ��____________________��
��5��˵������Ʒ���������֮��____________________________________________��
���𰸡�©�����ձ��������� ����������NaNO3����NO![]() �� ȡ�����ں���ϲ���Һ�������Թ��У�����Թ��еμ�BaCl2��Һ����������˵��SO
�� ȡ�����ں���ϲ���Һ�������Թ��У�����Թ��еμ�BaCl2��Һ����������˵��SO![]() �ѳ��� ��ȥ������BaCl2(��Ba
�ѳ��� ��ȥ������BaCl2(��Ba![]() ) Ba2++CO
) Ba2++CO![]() =BaCO3�� �ڲ�����ʱ���������Na2CO3��Һ������в����ܳ�ȥ
=BaCO3�� �ڲ�����ʱ���������Na2CO3��Һ������в����ܳ�ȥ
��������
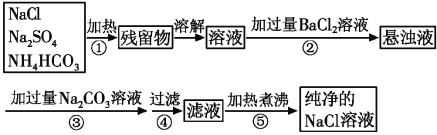
���������ơ�̼����淋��Ȼ��ƹ��壬�����̿�֪�����з������������·ֽⷴӦ���ֽ��Ĺ�������ˮ�����Ȼ�����Ӧ��ȥ��������ӣ�������̼���Ƴ�ȥ�����ı����ӣ����˺���Һ�к�NaCl��̼���ƣ��������ȥ̼���ƺ�����ΪNaCl�������õ�������NaCl���Դ������
���������ơ�̼����淋��Ȼ��ƹ��壬�����̿�֪�����з������������·ֽⷴӦ���ֽ��Ĺ�������ˮ�����Ȼ�����Ӧ��ȥ��������ӣ�������̼���Ƴ�ȥ�����ı����ӣ����˺���Һ�к�NaCl��̼���ƣ��������ȥ̼���ƺ�����ΪNaCl�������õ�������NaCl��
��1��������Ϊ���ˣ�����IJ��������У�©�����ձ�����������
��2�������ᱵ��Һ������������������ӣ����Գ�ȥ���ʴ�Ϊ������������NaNO3����NO![]() ���ӣ���
���ӣ���
��3�����в����ں��ж���Һ��SO42-�ѳ����ķ���Ϊȡ�����ں���ϲ���Һ�������Թ��У�����Թ��еμ�BaCl2��Һ����������˵��SO![]() �ѳ�����
�ѳ�����
��4�������۷�����Ӧ�Ļ�ѧ����ʽΪBaCl2+Na2CO3�TBaCO3��+2NaCl����Ŀ��Ϊ��ȥ������BaCl2(��Ba![]() ����)����Ӧ�����ӷ���ʽΪBa2++CO
����)����Ӧ�����ӷ���ʽΪBa2++CO![]() =BaCO3����
=BaCO3����
��5��������������֪������Ʒ���������֮������ȷ�ķ���Ϊ�ڲ�����ʱ�����Na2CO3��Һ��в����ܳ�ȥ������ڲ�����ʱ���������Na2CO3��Һ������в����ܳ�ȥ��

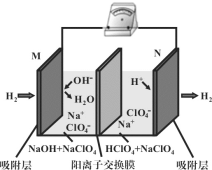
 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�����Ŀ��������ȾԽ��Խ��Ϊ���ǹ�ע�����⣬�����е�NOx�����ѳ�(������)֮������ŷš�
(1)CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺�� ��ӦCO(g)��H2O(g) ![]() H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯�����ʾ��
H2(g)��CO2(g)��ƽ�ⳣ�����¶ȵı仯�����ʾ��
�¶�/�� | 400 | 500 | 830 | 1 000 |
ƽ�ⳣ��K | 10 | 9 | 1 | 0.6 |
�ٴ��ϱ������ƶϣ��˷�Ӧ��__________(����������������)�ȷ�Ӧ��
����830 ���£�����ʼʱ������ܱ������г���CO��H2O��Ϊ1 mol����ﵽƽ���CO��ת����Ϊ________��
(2)������β��ϵͳ��װ�ô�ת����������Ч����NOx��CO���ŷš�
��֪����2CO(g)��O2(g) ![]() 2CO2(g) ��H��566.0 kJ��mol1
2CO2(g) ��H��566.0 kJ��mol1
��N2(g)��O2(g) ![]() 2NO(g) ��H��+180.0 kJ��mol1
2NO(g) ��H��+180.0 kJ��mol1
��2NO(g)��O2(g) ![]() 2NO2(g) ��H��116.5 kJ��mol1
2NO2(g) ��H��116.5 kJ��mol1
�ش��������⣺
��CO��ȼ����Ϊ _________����1 mol N2(g)��1 mol O2(g) �����л�ѧ������ʱ�ֱ���Ҫ����946 kJ��498 kJ����������1 mol NO(g) �����л�ѧ������ʱ�����յ�����Ϊ_________kJ��
��д��CO��NO2��ԭΪ���ʷ�Ӧ���Ȼ�ѧ����ʽΪ ____________________
(3)�����������ϵĴ�ת����������������CO��NO2��ԭΪ���ʷ�Ӧ����һ���¶��£���һ������CO��NO2����2L�̶��ݻ��������У��ش��������⣺
����˵���÷�Ӧ�ﵽƽ��״̬����_____________(����ĸ���)��
A.2����(NO2)=����(N2)
B. ��������ƽ����Է����������ֲ���
C.������ѹǿ���ٱ仯
D. ��H���ֲ���
E�����������ܶȲ��ٱ仯
�ڴӷ�Ӧ��ʼ��5min��������0.08mol N2����5min����(CO)=___molL1min1��
��25minʱ������Ũ�ȱ仯��ͼ��ʾ����ı������������___________(����ĸ���)��
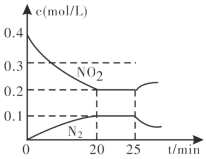
A.���������
B.����NO2��Ũ��
C.�����¶�
D.�����¶�