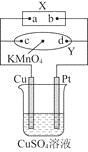
��Ŀ����
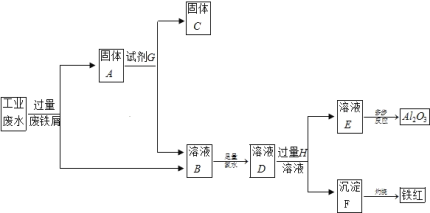
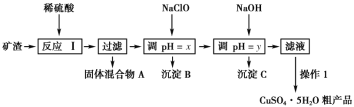
����Ŀ��ij�����ijɷ�ΪCu2O��Al2O3��Fe2O3��FeO��SiO2����ҵ���øÿ�����ȡͭ�͵����IJ���������ͼ��
��֪����Cu2O��2H+=Cu��Cu2+��H2O��
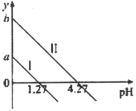
�ڲ���������������������ʽ����ʱ��Һ��pH���±���ʾ��
������ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
��ʼ����pH | 5.4 | 4.0 | 2.7 | 5.8 |
������ȫpH | 6.7 | 5.2 | 3.7 | 8.8 |
��1��Ϊ�˼ӿ췴Ӧ�������ʣ����Բ�ȡ�Ĵ�ʩ��__(��д1��)��
��2����������A�еijɷ���__��
��3����Ӧ����ɺ���Ԫ�صĴ�����ʽΪ__(�����ӷ���)����������ӳ��õķ���֮һ�ǣ�ȡ�����������ӵ���Һ���Թ��У��μӼ������軯����Һ�������������д���÷�Ӧ�����ӷ���ʽ__��
��4������1��Ҫ������__��__��__��ϴ��CuSO4��5H2O�ֲ�Ʒ�����ô���ˮϴ�����ñ�ˮϴ�ӡ�ԭ����__��
��5����NaClO��pH�������ɳ���B����������������Ϣ��������BΪ__���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__��
��6����NaOH��pH�������ɳ���C����������������Ϣ����y�ķ�ΧΪ__��
���𰸡��ʵ������¶ȣ����Ͻ��裻���������飻�ʵ����������Ũ�ȵ� SiO2��Cu Fe2+ 3Fe2++2[Fe(CN)6]3-=Fe3[Fe(CN)6]2�� ����Ũ�� ��ȴ�ᾧ ���� ��ˮ�ȿ���ϴȥ���������������ӣ��ֿ��Լ��پ������ʧ Fe(OH)3 1��2 5.2~5.4��5.2��pH<5.4
��������
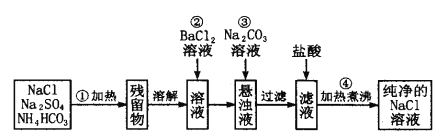
ij�����ijɷ�ΪCu2O��Al2O3��Fe2O3��FeO��SiO2������ϡ���ᷢ���ķ�ӦI��Cu2O+H2SO4=Cu+CuSO4+H2O��Al2O3+3H2SO4=Al2(SO4)3+3H2O��Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��FeO+H2SO4=FeSO4+H2O��Cu+ Fe2(SO4)3=CuSO4+2FeSO4��SiO2��ϡ�����Ӧ�������˵õ��Ĺ�������A�к�SiO2��Cu����Һ�к�CuSO4��Al2(SO4)3��FeSO4�����ݲ���������������������ʽ����ʱ��Һ��pH����Һ�м���NaClO����pH=x��Fe2+ת����Fe(OH)3�������ټ���NaOH��pH=yʹAl3+ת����Al(OH)3��������ʱ�õ�����ҺΪCuSO4��Һ������ͭ��Һ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӵõ�CuSO4��5H2O�ֲ�Ʒ���ݴ˷���������֪ʶ���
��1��������������Ի�ѧ��Ӧ���ʵ�Ӱ�죬Ϊ�˼ӿ췴Ӧ�������ʣ����Բ�ȡ�Ĵ�ʩ�ǣ��ʵ������¶ȡ����Ͻ��衢���������顢�ʵ����������Ũ�ȵȡ�
��2�������ijɷ�ΪCu2O��Al2O3��Fe2O3��FeO��SiO2�����������֪�ٺ���AΪ��������ϡ���ᷢ���ķ�ӦI��Cu2O+H2SO4=Cu+CuSO4+H2O��Al2O3+3H2SO4=Al2(SO4)3+3H2O��Fe2O3+3H2SO4=Fe2(SO4)3+3H2O��FeO+H2SO4=FeSO4+H2O��Cu+ Fe2(SO4)3=CuSO4+2FeSO4��SiO2���������������ϡ�����Ӧ�����˵õ��Ĺ�������A�ijɷ���SiO2��Cu��
��3������������������������A����Cu����Ӧ����ɺ���Ԫ�صĴ�����ʽΪFe2+�������軯����Һ����Fe2+������ɫ��������Ӧ�����ӷ���ʽΪ3Fe2++2[Fe(CN)6]3-=Fe3[Fe(CN)6]2����
��4������1Ϊ��CuSO4��Һ�л��CuSO4��5H2O�ֲ�Ʒ����Ҫ����������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӣ�ϴ��CuSO4��5H2O�ֲ�Ʒ�����ô���ˮϴ�����ñ�ˮϴ�ӣ�ԭ���ǣ���ˮ�ȿ���ϴȥ���������������ӣ��ֿ��Լ��پ������ʧ��
��5�����ݷ�������NaClO����pH�������ɳ���B������NaClO����ǿ�����ԣ�Fe2+���л�ԭ�ԣ����Գ���BΪFe(OH)3���÷�Ӧ��FeԪ�صĻ��ϼ���+2������+3�ۣ�Fe2+Ϊ��ԭ����NaClOΪ��������ClԪ�صĻ��ϼ���+1�۽���-1�ۣ����ݵ�ʧ�����غ㣬�������뻹ԭ�����ʵ���֮��Ϊ1:2��
��6�����ݷ�������NaOH��pH��ʹAl3+��ȫ��������ȥ����Cu2+���γɳ��������ݲ���������������������ʽ����ʱ��Һ��pH��y�ķ�ΧΪ5.2��pH<5.4��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�