��Ŀ����
20���������ӷ���ʽ��ȷ���ǣ�������| A�� | �����������������ⸯʴʱ��������������������Fe-3e-�TFe3+ | |
| B�� | SO2����ͨ��Fe2��SO4��3��Һ�У�SO2+2H2O+2Fe3+�T2Fe2++4H++SO42- | |
| C�� | ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O $\frac{\underline{\;���\;}}{\;}$ 2Cu+O2��+4H+ | |
| D�� | Na2S��Һʹ��̪��죺S2-+2H2O?2OH-+H2S |
���� A��������ʧȥ2�����������������ӣ��������������ӣ�
B�����������������ӷ���������ԭ��Ӧ�����������Ӻ���������ӣ�
C���缫Ϊͭ������ͭ�ŵ磬����ͭ���ӵõ���������ͭ���ù���Ϊ��⾫��ͭ��
D�������ӵ�ˮ����̷ֲ����У�ˮ������ӷ���ʽ��Ҫ�ֱ�д����Ҫ�Ե�һ��Ϊ����
��� �⣺A�������������������ⸯʴʱ�����������������������������ӣ���ȷ�ĵ缫��ӦʽΪ��Fe-2e-�TFe2+����A����
B��SO2����ͨ��Fe2��SO4��3��Һ�У����߷���������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��SO2+2H2O+2Fe3+�T2Fe2++4H++SO42-����B��ȷ��
C����ͭ���缫���CuSO4��Һ������Ϊͭ���������ŵ��Ϊͭ����Ӧ���������������ù����൱�ڵ�⾫��ͭ����C����
D������Һ�У������Ӳ���ˮ�⣬��Һ��ʾ���ԣ������ܹ�ʹ��̪��죬���������ӵ�ˮ�ⷽ��ʽ��Ҫ�ֱ�д����Ҫд����һ����ˮ�⼴�ɣ���ȷ�����ӷ���ʽΪ��S2-+H2O?OH-+HS-����D����
��ѡB��
���� ���⿼�������ӷ���ʽ���жϣ�Ϊ�е��Ѷȵ����⣬ע���������ӷ���ʽ�����жϳ��÷������磺��鷴Ӧ�ܷ�������鷴Ӧ��������Ƿ���ȷ���������ʲ���Ƿ���ȷ���������������ʵ���Ҫ������ѧʽ������Ƿ�����غ��ϵ���磺�����غ�͵���غ�ȣ���DΪ�״��㣬ע�������ӵ�ˮ��ֲ����У�

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д���1����ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��C��s��+H2O��g��?CO��g��+H2��g����H=+131.3kJ•mol-1��
�ٸ÷�Ӧ�Է����е���������ǽϸ��¶ȣ�
����һ�����ݵ��ܱ������У�һ���¶��·���������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����BC ������Ӧ�ı�ţ���
A��CO��H2�����������ͬ
B��1mol H-H�����ѵ�ͬʱ����2mol H-O��
C�������е�ѹǿ����
D������H2O�����ʵ�������H2������
��2������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������У��ڴ��������½��з�Ӧ��
CO��g��+H2O��g��?H2��g��+CO2��g�����õ�����ж������ݣ�
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | ||
| CO | H2O | H2 | CO | ||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 |
�ڼ���ʵ��2������ƽ�ⳣ����ֵK=0.17��
��3��pKa��ʾ����������ʵ���ƽ�ⳣ���ĸ���������pKa=-lgKa���й�������ͼ��
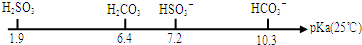
��0.01mol•L-1NaHSO3��Һ��pH=bl��0.01mol•L-1NaHCO3��Һ��pH=b2����b1��b2�����������������=������
����10mL 0.01mol•L-1��H2SO3��Һ�У��μ�0.0l mol•L-1KOH��Һ10mL����Һ�д���c��H+����c��OH-����������������K+��H2SO3��HSO3-��SO32-����Ũ���ɴ�С��˳��Ϊc��K+����c��HSO3-����c��SO32-����c��H2SO3����
| A�� | ����������Դ��ֲ�P��ӹ���Ʒ���������������ɫֲ��ͨ��������ý���ѧ��ת��Ϊ�������� | |
| B�� | �ð�ˮ������ȼú�����еĶ�����������������Ⱦ���ֿɵõ�����Ʒ����� | |
| C�� | ijС���о���Ա�ɹ��Ʊ�����������ҿ�����ӣ�IrO4+���������ģ��Ϊ ���ɴ˿��ж�����ҿԪ����+9�� ���ɴ˿��ж�����ҿԪ����+9�� | |
| D�� | �Ž��з����Ԫ��������Ԫ�ص����ԭ����������������Ԫ�������Ա仯�Ĺ����ų��˵�һ��Ԫ�����ڱ� |
N2��g��+3H2��g���T2NH3��g����H=-92.6kJ•mol-1
ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��
| ���� ��� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ�� | ��ƽ��ʱ��ϵ �����ı仯 | ||
| N2 | H2 | NH3 | |||
| �� | 1 | 3 | 0 | 2���� | ���� 46.3 kJ |
| �� | 0.4 | 1.2 | 1.2 | / | Q��Q��0�� |
| A�� | ��ƽ��ʱ������������NH3�����ʵ���Ũ����� | |
| B�� | �������з�Ӧ�ӿ�ʼ���մ�ƽ��ʱ��NH3��ʾ�ķ�Ӧ����Ϊv��NH3��=$\frac{1}{30}$mol•L-1•s-1 | |
| C�� | �����������Ϊ0.3 L�����ƽ��ʱ�ų�����������46.3kJ | |
| D�� | �������з�Ӧ�ﵽƽ��ʱ���յ�����ΪQ |
| A�� | ��״���£�22.4L��������ˮת�Ƶ�����ΪNA | |
| B�� | 1L 0.1mol/L����������Һ�к��������ӵ���ĿΪ0.2NA | |
| C�� | �����ϳ�����ͨ��amolN2��3amolH2�Ļ��������ַ�Ӧ������N-H��Ϊ6aNA | |
| D�� | �������ͭ��Һʱ������������22.4L����������壬��ϵ��ת�Ƶ�����Ϊ4NA |
| A�� | �õζ���װ��ҺʱӦ��ˮϴ���ñ�Һ��ϴ��װ���Һ | |
| B�� | ��ƿ������Ӧ����ʱһ�����ܼ��� | |
| C�� | ����ʱ�¶ȼ�ˮ������Ը�������ƿ֧�ܿ� | |
| D�� | ��ȡʵ������Һ©��ʱ��Ӧ�ر��䲣���������� |
| A�� | ����Һ�У�K+��Fe2+��C6H5OH��Br-���Դ������� | |
| B�� | ��KI��Һ��Ӧ�����ӷ���ʽ��2Fe3++2I-=2Fe2++I2 | |
| C�� | ��Ba��OH��2��Һ��Ӧ�����ӷ���ʽ��Fe3++SO42-+Ba2++3OH-=Fe��OH��3��+BaSO4�� | |
| D�� | 1L0.1mol/L����Һ��������Ca��ַ�Ӧ������11.2gFe |
 ��A��B��C��D��E���ֶ�����Ԫ�أ�A��Cͬ���ڣ�B��Dͬ���壬E������Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�A�ǹ��ɿ������ʯ����Ҫ�ɷֵ�Ԫ�أ�C��D���������ӻ�����C3D2��A�ĵ�����E�ĵ����ڳ����¾Ϳɷ�����Ӧ�����ɻ�����F���ݴ˻ش��������⣺
��A��B��C��D��E���ֶ�����Ԫ�أ�A��Cͬ���ڣ�B��Dͬ���壬E������Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�A�ǹ��ɿ������ʯ����Ҫ�ɷֵ�Ԫ�أ�C��D���������ӻ�����C3D2��A�ĵ�����E�ĵ����ڳ����¾Ϳɷ�����Ӧ�����ɻ�����F���ݴ˻ش��������⣺ ��A���ʼ�����������������ԭ�Ӿ��壨������ͣ�
��A���ʼ�����������������ԭ�Ӿ��壨������ͣ�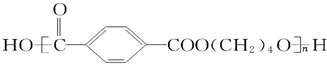 �䵥����
�䵥����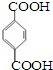 ��HO-CH2CH2CH2CH2-OH��
��HO-CH2CH2CH2CH2-OH��