��Ŀ����
8����ͬ�¶��£������Ϊ0.25 L�����������ܱ������з������淴Ӧ��N2��g��+3H2��g���T2NH3��g����H=-92.6kJ•mol-1
ʵ���÷�Ӧ����ʼ���ﵽƽ��ʱ���й��������±���ʾ��
| ���� ��� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ�� | ��ƽ��ʱ��ϵ �����ı仯 | ||
| N2 | H2 | NH3 | |||
| �� | 1 | 3 | 0 | 2���� | ���� 46.3 kJ |
| �� | 0.4 | 1.2 | 1.2 | / | Q��Q��0�� |
| A�� | ��ƽ��ʱ������������NH3�����ʵ���Ũ����� | |
| B�� | �������з�Ӧ�ӿ�ʼ���մ�ƽ��ʱ��NH3��ʾ�ķ�Ӧ����Ϊv��NH3��=$\frac{1}{30}$mol•L-1•s-1 | |
| C�� | �����������Ϊ0.3 L�����ƽ��ʱ�ų�����������46.3kJ | |
| D�� | �������з�Ӧ�ﵽƽ��ʱ���յ�����ΪQ |
���� A�����º����£����а���ѧ������ת������ߣ�����n��N2��=1mol��n��H2��=3mol����ƽ������ȫ��Чƽ�⣬�ݴ��жϣ�
B���ɴ�ƽ��ʱ�ٷų�����Ϊ46.3kJ������ƽ��ʱNH3�����ʵ������ٸ���v=$\frac{\frac{��n}{V}}{��t}$����v��NH3����
C����ƽ���ƶ��ĽǶȱȽϷ�Ӧ�ų���������46.3kJ�Ĺ�ϵ��
D���ɴ�ƽ��ʱ�ٷų�����Ϊ46.3kJ������ƽ��ʱN2��H2��NH3�����ʵ������������Ƿ���ƽ��״̬�������ĸ�������У��жϢ������Ȼ��Ƿ��ȣ�
��� �⣺A�����º����£����а���ѧ������ת������ߣ�n��N2��=0.4mol+1.2mol��$\frac{1}{2}$=1mol��n��H2��=1.2mol+1.2mol��$\frac{3}{2}$=3mol����ƽ������ȫ��Чƽ�⣬������������NH3�����ʵ���Ũ����ȣ���A��ȷ��
B��2min��ƽ��ʱ���ٷų�����Ϊ46.3kJ����ƽ��ʱXY3�����ʵ���Ϊ2mol��$\frac{46.3kJ}{92.6kJ}$=1mol����v��NH3��=$\frac{\frac{1mol}{0.25L}}{120s}$=$\frac{1}{30}$mol•L-1•s-1����B��ȷ��
C�������������Ϊ0.3L����Сѹǿƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʽ��ͣ���ƽ��ʱ�ų�������С��46.3kJ����C����
D���������зų�46.3kJ�����������ɰ��������ʵ���Ϊ��2mol��$\frac{46.3kJ}{92.6kJ}$=1mol����
?N2��g��+3H2��g��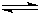 2NH3��g��
2NH3��g��
��ʼ��1mol 3mol 0
ת����0.5mol 1.5mol 1mol
ƽ�⣺0.5mol 1.5mol 1mol
���������㣬��֪ƽ��ʱ��������N2��H2��NH3�����ʵ����ֱ�Ϊ0.5mol��1.5mol��1mol�������Ϊ��ȫ��Чƽ�⣬���ԣ�ƽ��ʱ��������N2��H2��NH3�����ʵ���Ҳ�ֱ�Ϊ0.5mol��1.5mol��1mol����֪�ڵķ�Ӧ���淴Ӧ������У���Ӧ������Ҫ��������ΪQ����D��ȷ��
��ѡ��C��
���� ���⿼�黯ѧƽ���ƶ����⡢��Чƽ�⡢��Ӧ�ȵ����⣬��Ŀ�Ѷ��еȣ�ע��C����ݼ��������ע��ӵ�Чƽ��ĽǶȽ��Ҳ��������ƽ�ⳣ�����

| A�� | ͨ������CO2�����Һ�У�Na+��SO32-��CH3COO-��HCO3- | |
| B�� | ��ɫ��Һ�У�Mg2+��MnO4-��SO42-��K+ | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012����Һ�У�NH4+��Fe2+��NO3-��Cl- | |
| D�� | c��ClO-��=1.0 mol•L-1����Һ��Na+��SO32-��S2-��SO42- |
| A�� | ��ʳ�׳�ȥ��ˮƿ�е�ˮ����CO32-+2CH3COOH�T2CH3COO-+CO2��+H2O | |
| B�� | Al����NaOH��Һ��Al+2OH-�TAlO2-+H2�� | |
| C�� | Cl2����ˮ��Cl2+H2O�TH++Cl-+HClO | |
| D�� | NaHSO4��Һ��Ba��OH��2��Һ��Ӧ�����ԣ�H++SO42-+Ba2++OH-�TBaSO4��+H2O |
| A�� | �����������������ⸯʴʱ��������������������Fe-3e-�TFe3+ | |
| B�� | SO2����ͨ��Fe2��SO4��3��Һ�У�SO2+2H2O+2Fe3+�T2Fe2++4H++SO42- | |
| C�� | ��ͭ���缫���CuSO4��Һ��2Cu2++2H2O $\frac{\underline{\;���\;}}{\;}$ 2Cu+O2��+4H+ | |
| D�� | Na2S��Һʹ��̪��죺S2-+2H2O?2OH-+H2S |
| A�� | ��״���£���0.1mol•L -1�Ĵ�����Һ�м�������ƾ��壬�����Һ��pH=7�������Һ��c��Na+����c��CH3COO-�� | |||||||||||
| B�� | �����±����йع��ۼ��ļ��ܣ�
 ��g��+3H2��g���� ��g��+3H2��g���� ��g���ġ�H=-348kJ•mol-1 ��g���ġ�H=-348kJ•mol-1 | |||||||||||
| C�� | һ�����ĸ���NH4Fe��SO4��2��Һ����μ���Ba��OH��2��Һ����ijһʱ�̣���Ӧ�����ӷ���ʽ������2Fe3++3SO42-+3Ba2++6OH-�T3BaSO4��+2Fe��OH��3�� | |||||||||||
| D�� | ��֪��25��C��Ksp��BaSO4��=1��10-10������¶��µı���BaSO4��Һ�м���һ�����������ƻ��Ȼ��������ʹKsp��BaSO4������ |
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��Ũ��Ϊ0.1mol•L-1��MgCl2��CuCl2�����Һ����μ��백ˮ | �ȳ�����ɫ���� | Ksp[Mg��OH��2]��Ksp[Cu��OH��2] |
| B | ��������NaOH��Һ���Ⱥμ�AgNO3��Һ | δ���ֵ���ɫ���� | ������δˮ�� |
| C | �ýྻ��˿պȡ��Һ������ɫ��Ӧ | ����ʺ�ɫ | ԭ��Һ�в���K+ |
| D | ij���������ᣬ������ɫ��ζ����ͨ�����ʯ��ˮ | ����� | ˵��������̼���� |
| A�� | A | B�� | B | C�� | C | D�� | D |

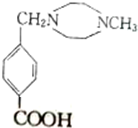 ��
��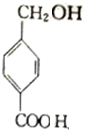 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$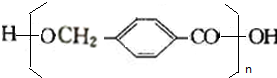 +��n-1��H2O
+��n-1��H2O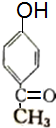 +2Br2��
+2Br2�� +2HBr��
+2HBr��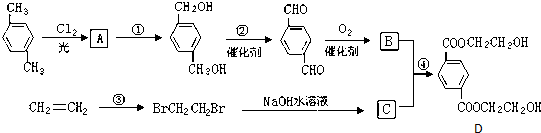
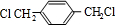 ��
��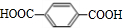 ��HOCH2CH2OH��
��HOCH2CH2OH��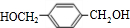 +2NaCl��ˮ�⣨ȡ������Ӧ��
+2NaCl��ˮ�⣨ȡ������Ӧ��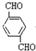 ��������Һ��Ӧ�Ļ�ѧ����ʽOHCC6H4CHO+4Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$NH4OOCC6H4COONH4+4Ag��+6NH3��+2H2O��
��������Һ��Ӧ�Ļ�ѧ����ʽOHCC6H4CHO+4Ag��NH3��2OH$\stackrel{ˮԡ����}{��}$NH4OOCC6H4COONH4+4Ag��+6NH3��+2H2O��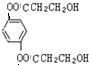 ��������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
��������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��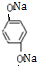 +2HOCH2CHCOONa��
+2HOCH2CHCOONa��