题目内容
19.(1)水的电离方程式为:H2O?H++OH-,或2H2O?H3O++OH-.(2)水的离子积表示式为:Kw=c(H+)×c(OH-),常温下该数值为:1×10-14.
现有下列物质:HNO3、NH3•H2O、KCl、Al2(SO4)3、NaHCO3、CuSO4、NH4Cl、Ba( NO3)2.
(3)其中能抑制水的电离的物质有HNO3、NH3•H2O;
(4)能促进水的电离的物质有Al2(SO4)3、NaHCO3、CuSO4、NH4Cl;
(5)不会破坏水的电离平衡的物质有KCl、Ba( NO3)2;
(6)从上述变化中得出的规律是:酸和碱会抑制水的电离,能发生水解的盐能促进水的电离.
分析 (1)水为弱电解质,能电离出氢离子和氢氧根离子;
(2)水的离子积常数为氢离子与氢氧根离子浓度的乘积,常温下Kw=10-14;
(3)酸碱抑制水的电离;
(4)能水解的盐促进水的电离;
(5)酸、碱或水解的盐破坏水的电离平衡.
解答 解:(1)水为弱电解质,电离出氢离子(或者水合氢离子)和氢氧根离子,电离方程式为H2O?H++OH-或2H2O?H3O++OH-,故答案为:H2O?H++OH-;2H2O?H3O++OH-;
(2)水的离子积常数为:Kw=c(H+)×c(OH-),常温下该数值=1×10-14,故答案为:Kw=c(H+)×c(OH-);1×10-14;
(3)抑制水的电离的物质是硝酸和一水合氨,故答案为:HNO3、NH3•H2O;
(4)Al2(SO4)3为强酸弱碱盐、NaHCO3为强碱弱酸盐、CuSO4为强酸弱碱盐、NH4Cl为强酸弱碱盐,均水解,促进水的电离,故答案为:Al2(SO4)3、NaHCO3、CuSO4、NH4Cl;
(5)KCl、Ba( NO3)2为强酸强碱盐,不能水解,不破坏水的电离平衡,故答案为:KCl、Ba( NO3)2;
(6)由以上得出的结论是:酸和碱会抑制水的电离,能发生水解的盐能促进水的电离,故答案为:酸和碱会抑制水的电离,能发生水解的盐能促进水的电离.
点评 本题主要考查的是水的电离以及其影响因素,酸或碱均抑制水的电离,能水解的盐促进水的电离,难度不大.

练习册系列答案
相关题目
9.由短周期前10号元素组成的物质T和X,有如图所示的转化.X不稳定,易分解.为使得下列转化能够成功进行,方框内不可能加入的反应试剂是( )
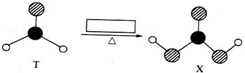

| A. | 新制Cu(OH)2悬浊液 | B. | 溴水 | C. | 酸性KMnO4溶液 | D. | NaOH溶液 |
7.下列实验中,不能观察到明显变化的是( )
| A. | 把一段打磨过的镁带放入少量冷水中 | |
| B. | 把 Cl2通入FeCl2溶液中 | |
| C. | 把绿豆大的钾投入水中 | |
| D. | 氢氟酸滴到玻璃上 |
14.下列关于物质性质变化的比较,不正确的是( )
| A. | 酸性强弱:H2SiO3<H2CO3<HNO3 | B. | 原子半径大小:Na>S>O | ||
| C. | 碱性强弱:KOH>NaOH>LiOH | D. | 非金属性强弱:I>Cl>F |
9.下列分子中的中心原子的杂化类型为sp2杂化的是( )
| A. | BeCl2 | B. | NH3 | C. | BF3 | D. | H2O |

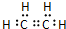 ,分子中含有的共价键类型有非极性键、极性键(填极性键或非极性键). C中含官能团名称羟基;
,分子中含有的共价键类型有非极性键、极性键(填极性键或非极性键). C中含官能团名称羟基; ,反应类型:加聚反应.⑤CH2=CH2+H2O
,反应类型:加聚反应.⑤CH2=CH2+H2O ; O22+中σ键数目和π键数目之比为1:2.
; O22+中σ键数目和π键数目之比为1:2.
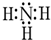 ,分子间存在氢键.
,分子间存在氢键.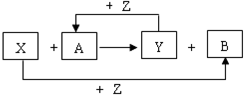 X、Y、Z为常见的三种单质,Z是绿色植物光合作用的产物之一,A、B为常见化合物.它们在一定条件下可发生如图所示的反应(均是在非溶液中进行的反应):
X、Y、Z为常见的三种单质,Z是绿色植物光合作用的产物之一,A、B为常见化合物.它们在一定条件下可发生如图所示的反应(均是在非溶液中进行的反应): ;
; 或
或 ;
;