��Ŀ����
��10�֣���һ������SO2�ͺ�0.7mol�����Ŀ���(����CO2)����0.5 L�ܱ������ڣ�550��ʱ���ڴ��������·�����Ӧ��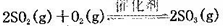 ������Ӧ���ȣ������n��O2����ʱ��ı仯���±�
������Ӧ���ȣ������n��O2����ʱ��ı仯���±�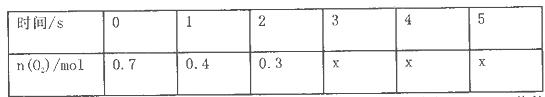
��Ӧ�ﵽ5s�������еĻ������ͨ������NaOH��Һ���������������22. 4L�������Ϊ��״���µ���������ٽ�ʣ������ͨ������ûʳ����ļ�����Һ����O2�����������ּ�����5.6L�������Ϊ��״���µ��������
��ش��������⣺
��1����O2��ʾ��0-ls�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ__________________��
��2��O2��ƽ��Ũ��c (O2)=____________________________��
��3�� 4sʱ��SO2����������____________������ڡ�����С�ڡ����ڡ���O2���������ʡ�
��4����÷�Ӧ�ﵽƽ��ʱSO2��ת������________���ðٷ�����ʾ����
��5������ƽ���̨������SO3��5%ͨ�������BaCl2��Һ�����ɳ���_______�ˣ�����������һλС������
��1��0.6mol/(L��s)
��2��0.5mol/L
��3������
��4��90%
��5��10.5
���������������1��0-ls��O2�����ʵ�������0.7-0.4=0.3mol��Ũ�ȼ���0.3mol/0.5L=0.6mol/L��������O2��ʾ��0-ls�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ0.6mol/L/1s=0.6mol/(L��s)
��2�����������֪�����������Ƶķ�Ӧ���Ƕ���������������Ļ�����壬���ߵ����ʵ���֮���뿪ʼ����Ķ�����������ʵ�����ͬ�����Ի������ͨ������������Һ������٣���״����22.4L��˵����ʼͨ��Ķ�����������ʵ�����1mol��ʣ������ͨ������ûʳ����ļ�����Һ����O2�����������ּ�����5.6L����״������˵��ʣ������5.6L�����ʵ�����0.25mol��Ũ����5.6L/22.4L/mol/0.5L=0.5mol/L������ѹǿ��ƽ��Ũ����0.5mol/L��
��3��4sʱ����Ӧ�Ѵ�ƽ�⣬v��(SO2)=2v����O2��������SO2���������ʴ���O2���������ʡ�
��4��������ƽ�����ʵ�����0.25mol������������0.7mol-0.25mol=0.45mol���������Ķ�������0.45mol��2=0.9mol�����ݣ�2���ķ�����֪����������ij�ʼ����1mol�����Ը÷�Ӧ�ﵽƽ��ʱSO2��ת������0.9mol/1mol��100%=90%��
��5����������е�����������BaCl2��Һ��Ӧ�������ᱵ������ͬ���ɼ��������������������ʵ�����0.9mol����5%��BaCl2��Һ��Ӧ���������ᱵ0.045mol��������10.485g������һλС����10.5g��
���㣺�����������Ĵ�������Ӧ����Ӧ���ʵļ������жϣ���ѧ��Ӧ���йؼ���

��H2(g)��Br2(g)��������ܱ������������·�����ӦH2��g��+Br2(g)  2HBr��g����H��0��ƽ��ʱBr2(g)��ת����Ϊa������ʼ������ͬ�������½���������Ӧ��ƽ��ʱBr2(g)��ת����Ϊb��a��b�Ĺ�ϵ��
2HBr��g����H��0��ƽ��ʱBr2(g)��ת����Ϊa������ʼ������ͬ�������½���������Ӧ��ƽ��ʱBr2(g)��ת����Ϊb��a��b�Ĺ�ϵ��
| A��a��b | B��a=b | C��a��b | D����ȷ�� |
������16�֣�
��1��Ϊ�˼�������β����ɵĴ�����Ⱦ�����ǿ�ʼ̽������NO��CO��һ��������ת��Ϊ����������E��F�ķ���(��֪�÷�Ӧ��H<0). ��2 L�ܱ������м���һ����NO��CO�����¶ȷֱ���T1��T2ʱ����ø�����ƽ��ʱ���ʵ������±���
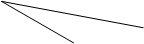 ���� ����T/�� n/mol | NO | CO | E | F |
| ��ʼ | 0.100 | 0.100 | 0 | 0 |
| T1 | 0.020 | 0.020 | 0.080 | 0.040 |
| T2 | 0.010 | 0.010 | 0.090 | 0.045 |
�������ϱ����ݣ�д��NO��CO��Ӧ�Ļ�ѧ����ʽ .
�ڸ��ݱ��������жϣ��¶�T1��T2�Ĺ�ϵ��(�����)__________��
A��T1>T2 B��T1<T2 C��T1=T2 D�����Ƚ�
��2����֪��4NH3(g) + 3O2(g) = 2N2(g) + 6H2O(g); ��H= - 1266.8 kJ/mol
N2(g) + O2(g) =" 2NO(g)" ; ��H =" +" 180.5kJ/mol��
���������Ȼ�ѧ����ʽΪ________________________________________��
��3��500���£���A��B���������о������ϳɰ��ķ�Ӧ�������̶�������������������ƶ���

���ϳɰ�������B�д�ƽ��ʱ��������к���1.0molN2��0.4molH2��0.4molNH3����ʱ�ݻ�Ϊ2.0L����������µ�ƽ�ⳣ��Ϊ___________�������¶Ⱥ�ѹǿ���䣬���������ͨ��0.36molN2��ƽ�⽫___________������������������ƶ���
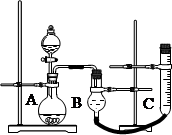
��4������ͬ���ܱ������У��÷�����ͷ������Ƶõ�����Cu2O�ֱ���д��ֽ�ˮ��ʵ�飺
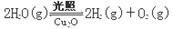 ��H >0
��H >0ˮ������Ũ�ȣ�mol��L��1����ʱ��t (min)�仯���±���
| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
��5�������о����֣��ø�Ĥ��ⷨ���Դ�����Ũ����ȩ��ˮ��ԭ����ʹ�ö��Ե缫����ȩ-Na2SO4��ҺΪ�������Һ����ȩ�ֱ�����������ת��Ϊ�Ҵ������ᡣ
�ܷ�ӦΪ:2CH3CHO+H2O
 CH3CH2OH+CH3COOH��
CH3CH2OH+CH3COOH�������У��������ֱ�����������Ҵ��⣬��������ɫ���壬�����缫��Ӧ�ֱ�Ϊ��
4OH��-4e���TO2��+2H2O�� ��
(12��).
��1.0 L�ܱ������з���0.10molA(g)����һ���¶Ƚ������·�Ӧ:
A(g) B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
B(g)��C(g) ��H=+85.1kJ��mol��1�����ȷ�Ӧ��
��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100kPa | 4.91 | 5.58 | 6.32 | 7.31 | 8.54 | 9.50 | 9.52 | 9.53 | 9.53 |
�ش���������:
��1�������A��ƽ��ת���ʣ�Ӧ��ȡ�Ĵ�ʩΪ�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��2������ѹǿP����ʼѹǿP0���㷴Ӧ��A��ת���ʦ�(A)�ı���ʽΪ�ߣߣߣߣߣߣߡ�ƽ��ʱA ��ת����Ϊ�ߣߣߣߣ�.
��3�� ������ѹǿp����ʼѹǿp0��ʾ��Ӧ��ϵ�������ʵ���n���ͷ�Ӧ��A�����ʵ���n��A����
n����_______mol��n��A����_______mol��
���±�Ϊ��Ӧ��AŨ���뷴Ӧʱ������ݣ�����a�� _______________
| ��Ӧʱ��t/h | 0 | 4 | 8 | 16 |
| C��A��/��mol��L-1�� | 0.10 | a | 0.026 | 0.0065 |
�����÷�Ӧ�з�Ӧ���Ũ��c��A���仯��ʱ��������t���Ĺ��ɣ��ó��Ľ����ǣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣ��ɴ˹����Ƴ���Ӧ��12hʱ��Ӧ���Ũ��c��A��Ϊ_______mol��L-1
��15�֣�̫���ܵ�������ù��ЧӦʵ�������仯��һ������װ�ã�Ŀǰ����õ�����Ͷྦྷ����Ϊ�������ϡ��ߴ��ȵľ�����ͨ�����·�Ӧ��ã�
��Ӧ�٣��ϳ�¯����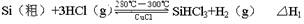
��Ӧ�ڣ���ԭ¯����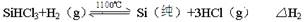
�й����ʵķе����±���ʾ��
| ���� | BCl3 | PCl3 | SiCl4 | AsCl3 | AlCl3 | SiHCl3 |
| �е� | 12��1 | 73��5 | 57��0 | 129��4 | 180�������� | 31��2 |
��1��̫���ܵ�ص�����ת����ʽΪ ���ɺϳ�¯�еõ���SiHCl3�����������ס��顢�����Ȼ������ʣ������SiHCl3�ķ����� ��
��2���������෴Ӧ����ij���(B)��ƽ��ѹǿ(PB)�������ʵ���Ũ��(cB)Ҳ�ɱ�ʾƽ�ⳣ��������KP������Ӧ�ٵ�KP�� ��
��3�����ڷ�Ӧ�ڣ���0��1Mpa�£���ͬ�¶Ⱥ�������ȣ�H2/SiHCl3����SiHCl3ʣ������Ӱ�����±���ʾ��

�ٸ÷�Ӧ�ġ�H2 0���>������<������=����
�ڰ��������5:1Ͷ�뻹ԭ¯�У���Ӧ��4minʱ���HCl��Ũ��Ϊ0��12mol��L��1����SiHCl3�����ʱ���ڵķ�Ӧ����Ϊ ��
�۶��ϱ������ݽ��з��������¶ȡ���ȶ�ʣ������Ӱ���У���ԭ¯�еķ�Ӧ�¶�ѡ����1100�棬����ѡ��775�棬���е�һ��ԭ��������ͬ����£��¶ȶ�SiHCl3 ʣ������Ӱ�죬�������һԭ���� ��
��4�����ڷ�Ӧ�ڣ���1100���£���ͬѹǿ��������ȣ�H2/SiHCl3����SiHCl3ʣ������Ӱ����ͼ27��1��ʾ��
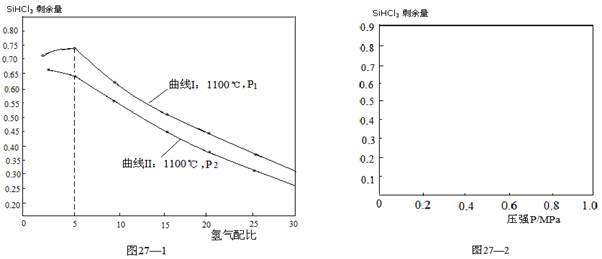
�� ͼ��P1 P2���>������<������=����
����ͼ27��2�л������������ͬ����£�1200���1100����¶��£�ϵͳ��SiHCl3ʣ������ѹǿ�仯�������仯����ʾ��ͼ��
ijѧ��Ϊ��̽��Zn�����ᷴӦ�����е����ʱ仯����100mLϡ�����м���������Zn�ۣ�����ˮ�������ռ���Ӧ�ų���H2��ʵ���¼���£��ۼ�ֵ����
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL��(�����) | 50 | 120 | 232 | 290 | 310 |
��1����һʱ��Σ�ָ0��1��1��2��2��3��3��4��4��5 min����Ӧ������� ������Ϊԭ���� ��
��2��4��5 minʱ��εķ�Ӧ������С������Ϊԭ���� ��
��3����2��3 minʱ������������Ũ�ȱ仯����ʾ�ķ�Ӧ���ʣ�������Һ������ֲ��䣩V��HCl��= ��
��4�������Ӧ̫���ң�Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ��������������������зֱ�����������ʣ�
A��H2O B��NaCl��Һ C��Na2CO3��Һ D��Cu�� E��CuSO4��ĩ
����Ϊ���е��ǣ����ţ� ��
 3C��g��+D��s����2���ӷ�Ӧ�ﵽƽ�⣬��ʱC��Ũ��Ϊ1��2 mol/L��
3C��g��+D��s����2���ӷ�Ӧ�ﵽƽ�⣬��ʱC��Ũ��Ϊ1��2 mol/L�� 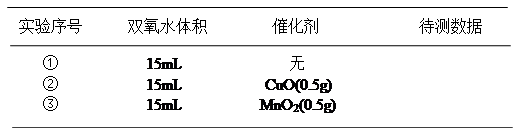
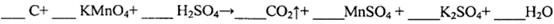
 ���õ������������ݣ�
���õ������������ݣ�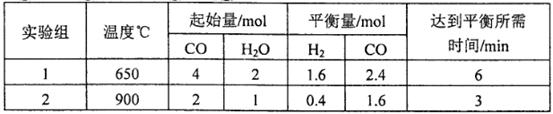
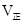 ____________
____________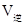 ���<������>������=������
���<������>������=������

 ��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ1��10
��CaCl2��Һ��Na2CO3��Һ��Ͽ��γ�CaCO3�������ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ1��10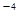 mol/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ__________mol/L��
mol/L�������ɳ�������CaCl2��Һ����СŨ��Ϊ__________mol/L��