��Ŀ����
����Ŀ���о����ϣ���ʦ��ͬѧ�Ǹ���ѡ����м��㣬������ȷ����
A.��ͬѧ��0.2 mol MgO�������㣬������Ϊ0.2 g
B.��ͬѧ��9.03��1023��O2�������㣬O2���ʵ���Ϊ1.5 mol
C.��ͬѧ����״���£�5.6 L ˮ�������������Ϊ 0.25 mol
D.��ͬѧ����30 mL0.5mol��L-1NaOH��Һ��ˮϡ�͵�500mL�������㣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ0.04mol��L-1
���𰸡�B
��������
A. MgO������Ϊ0.2mol��40g/mol=8g��ѡ��A����
B. O2���ʵ���Ϊ![]() =1.5mol��ѡ��B��ȷ��
=1.5mol��ѡ��B��ȷ��
C.��״���£�ˮ�������壬��������22.4L/mol�����㣬ѡ��C����
D.��30 mL0.5mol��L-1NaOH��Һ��ˮϡ�͵�500mL�������㣬ϡ�ͺ���Һ��NaOH�����ʵ���Ũ��Ϊ0.03mol��L-1��ѡ��D����
��ѡB��

����Ŀ����������SO2����һ���ڿռ������������ѧ�����ʿ�̽�������ܵ��㷺�о���һ�����塣
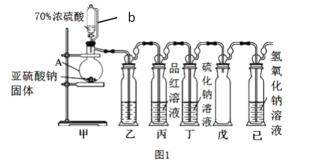
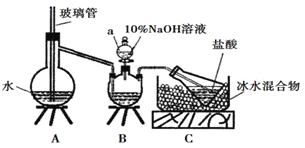
��.ij�о�С�������һ���Ʊ������� SO2 �������ʵ�װ�ã���ͼ 1 ��ʾ��
��1������ A ������______________������ b ������______________��
��2��װ���ҵ�������Ϊ�˹۲����� SO2�����ʣ���װ�����м�����Լ���______________��
��3����ʵ��ǰ��ͬѧ������ɣ���װ��û���ſ������������е� O2 ������ǿ�� SO2����� װ�ö��м�ʹ�л�������Ҳ����˵���� SO2 ���µġ�����д�� O2 �� Na2S ��Һ��Ӧ�Ļ�ѧ ��Ӧ����ʽ______________��
��Ϊ��һ������װ�ö��������������ԭ�����µ�ʵ��̽����ʵ����������������
��� | ʵ����� | ʵ������ |
1 | �� 10ml 1mol��L-1 �� Na2S ��Һ��ͨ O2 | 15min ����Һ�ų��ֻ��� |
2 | �� 10ml 1mol��L-1 �� Na2S ��Һ��ͨ SO2 | ��Һ�������ֻ�ɫ���� |
��ʵ�������֪����ʵ�������� Na2S ��Һ���ֻ��������� SO2 ���µġ�����Ϊ�ϱ�ʵ�� 1 ��Ӧ������ԭ�������______________��
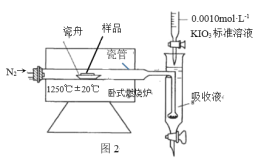
��.����ʯ����Ԫ�صIJⶨ����ʹ��ȼ�յ���������ԭ�����ڸ����½���Ʒ�е���Ԫ��ת�� Ϊ SO2 �� �� �� �� �� �� �� �� �� �� �� �� �� �� Һ Ϊ SO2 �� �� Һ �� �� SO2 �� �� �� ͬ ʱ �� 0.0010mol��L-1KIO3 ����Һ���еζ������װ����ͼ 2 ��ʾ��
[��������] ��ʵ����� 5min����Ʒ�е� S Ԫ�ض���ת��Ϊ SO2
��2IO3����5SO2��4H2O=8H����5SO42����I2
��I2��SO2��2H2=2I����SO42����4H��
��IO3����5I����6H��=3I2��3H2O
��4����ҵ�趨�ĵζ��յ�������______________��
��5��ʵ��һ���հ����飬������Ʒ����ʵ�飬5min �������ı�Һ���Ϊ V1mL
ʵ��������� 1g ��Ʒ�ٽ���ʵ�飬5min �������ı�Һ���Ϊ V2mL
�Ƚ����ݷ��� V1 ԶԶС�� V2���ɺ��Բ��� V1�� ��� V2 ��������
��� | 1 | 2 | 3 |
KIO3 ����Һ���/mL | 10.02 | 9.98 | 10.00 |
�÷�����ʯ��Ʒ����Ԫ�ص������ٷֺ���Ϊ______________��