��Ŀ����
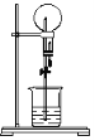
����Ŀ��ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣

��ش��������⣺
(1)װ��A��ʢ��Ũ���������������___________��
(2)��Ӧ��װ��B�з����������Ƿ�Ӧ�����ӷ���ʽΪ____________��װ��C�е�������___________��������SO2��________ �ԣ�װ��D��������____________��������Ӧ�Ļ�ѧ����ʽΪ_____________________��
(3)װ��E��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������____��
(4)Fװ�õ�������________________©����������________
���𰸡���Һ©�� Cl2+SO2+2H2O=2Cl-+SO42-+4H+ ��ɫ��ȥ ��ԭ�� ��������ɫ���� 2H2S+SO2=3S+2H2O �����Թܺ���Һ��ɫ�ָ���ɫ ���ն����SO2 ��ֹ����
��������
��ʵ��̽��SO2�����ʣ�����װ��ͼ��װ��A�Ʊ�SO2��װ��B��ʢ����ˮ����ˮ����ǿ�����ԣ���֤SO2�Ļ�ԭ�ԣ�װ��Cʢ�����Ը��������Һ����֤SO2�Ļ�ԭ�ԣ�װ��D��ʢ��H2S������SO2�������ԣ�װ��E��ʢ��Ʒ����Һ����֤SO2��Ư���ԣ���ΪSO2�ж�����Ⱦ�����������Ҫβ����������װ��F��ʢ��NaOH��Һ��
(1)����װ��ͼ��װ��A��ʢ��Ũ�������������Ϊ��Һ©����
(2)SO2�Ի�ԭ��Ϊ������ˮ����ǿ�����ԣ��ܽ�SO2�����������ӷ���ʽΪSO2��Cl2��H2O=2Cl����SO42����4H�������������Һ����ǿ�����ԣ��ܽ�SO2������SO42�������װ��C�е���������ɫ��ȥ��SO2Ҳ���������ԣ���H2S����SO2��2H2S=3S����2H2O���۲쵽�������Dz�������ɫ������
(3)SO2Ư������SO2����ɫ�л����ϣ��γɲ��ȶ�����ɫ���������ɫ�����������ָֻ�ԭ������ɫ��ʵ��������������Թܺ���Һ��ɫ�ָ���ɫ��
(4)SO2�ж�����Ⱦ��������Ҫβ��������װ��F�����������ն����SO2������©���������Ƿ�ֹ������